I want to give a big thanks to a colleague that passed me the following excellent work. My colleague prefers to remind anonymous. I have found the work very interesting and useful. I hope that you enjoy reading it as much as I have enjoyed.
Overview in the science behind the FTIR
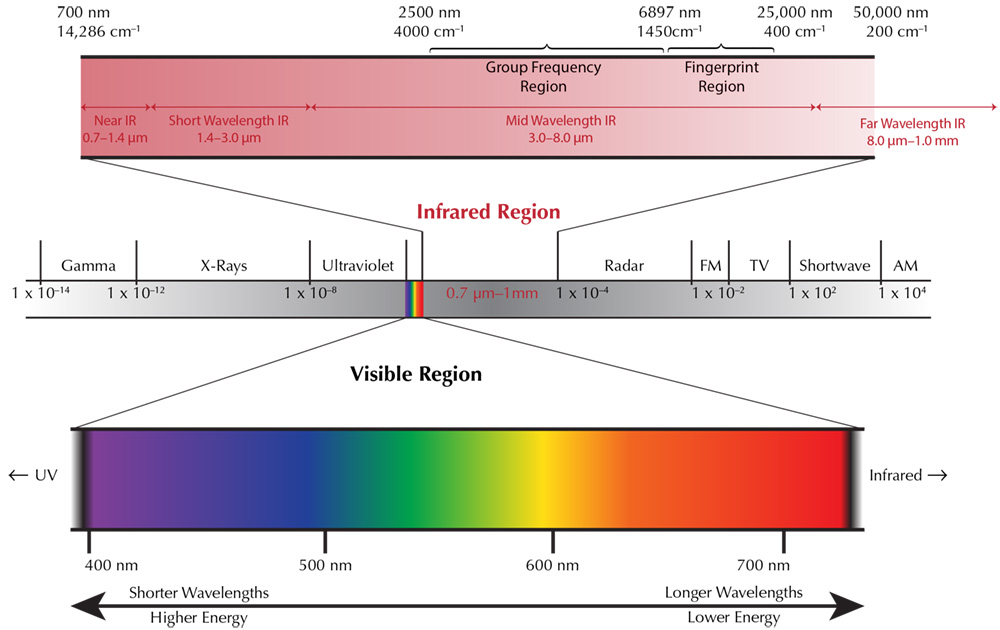 Source: FTIR in Gem Testing • FTIR Intrigue • Lotus Gemology | FTIR (Fourier Transform Infrared) Spectrometers are powerful tools used for material analysis. They work by passing infrared radiation through a sample, where some of the radiation is absorbed and some is transmitted. The range of Infrared region is 12800 ~ 10 cm-1 and can be divided into near-infrared region (12800 ~ 4000 cm-1), mid-infrared region (4000 ~ 200 cm-1) and far-infrared region (1000 ~ 50 cm-1). For FTIR the mid-infrared region is used. FTIR spectroscopy measures the infrared radiation absorbed by a material of interest. Each chemical molecule has a set of functional groups that absorb IR radiation at specific frequencies. Regardless of the overall structure of the chemical compound, these functional groups tend to absorb IR radiation at the same frequencies. This consistent absorption allows for the correlation between a molecule’s structure and the frequencies at which it absorbs IR radiation, facilitating the structural identification of unknown compounds. |
Fingerprint region
| The fingerprint region is called this because no two compounds produce the exact same absorption pattern in this region. This is useful when an unknown compound needs to be identified or to confirm the identity of a synthesised compound. The peaks in the fingerprint region are primarily due to the bending and stretching vibrations of carbon-carbon and carbon-hydrogen bonds which are common in all organic compounds. Each of these vibrations occurs at a different frequency that is unique to the chemical bond and compound, those frequencies happen to match the frequencies of light in the MIR region of the electromagnetic spectrum. In addition to identification, the fingerprint region can also provide information about the presence of impurities in a sample. If an unexpected peak appears in the fingerprint region, it may indicate the presence of an impure substance. This makes the fingerprint region not only useful for identification but also for quality control in chemical manufacturing. The fingerprint region, typically found between 1500 and 500 cm⁻1. | 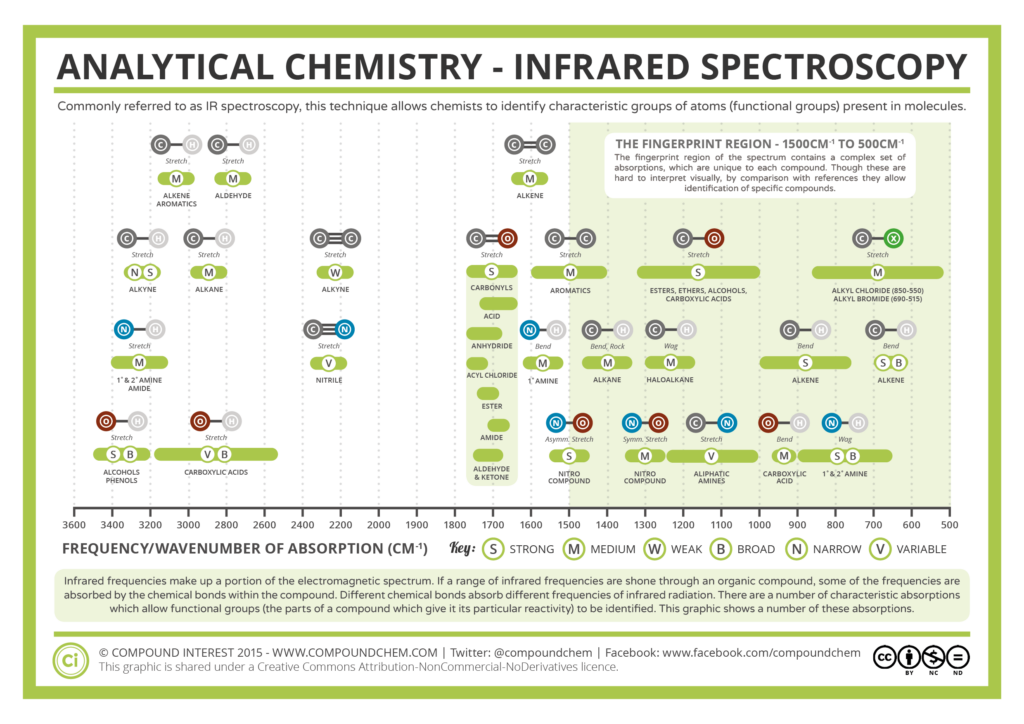 Source: Compound Interest: Analytical Chemistry – Infrared (IR) Spectroscopy (compoundchem.com) |
Attenuated Total Reflectance (ATR)
 Source: Attenuated Total Reflectance ATR-FTIR Spectroscopy Principles (specac.com) | IR Radiation passes through and Internal Reflection Element (IRE) crystal material. ATR uses a crystal with a high refractive index, such as diamond, zinc selenide (ZnSe), or germanium, that is placed in contact with the sample. When infrared radiation is directed into the crystal, it undergoes multiple internal reflections. The IR light interacts with the sample usually at a 45° o incident angle to the crystal/sample interface. During each reflection at the interface between the crystal and the sample, a portion of the infrared radiation penetrates a short distance into the sample (liquid or solid) as an evanescent wave. The sample absorbs some of the energy from the evanescent wave at specific wavelengths, depending on its molecular composition. This absorption modifies the intensity of the reflected infrared radiation. |
The modified infrared radiation exits the crystal and is collected by the detector, producing an IR spectrum that represents the sample’s absorption pattern.
Transmission
Transmission infrared (IR) spectroscopy is an analytical technique used to identify and quantify chemical compounds by measuring how they absorb infrared light as it passes through a sample.
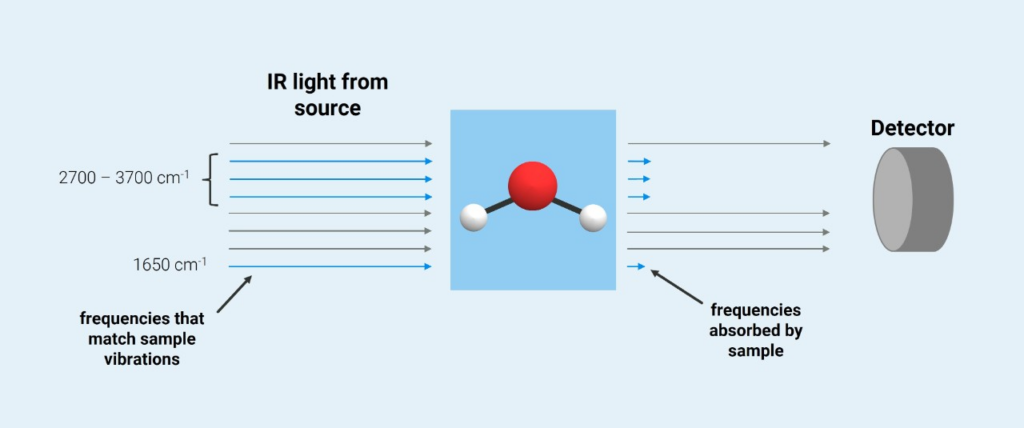
Source: Guide to FT-IR Spectroscopy | Bruker
KBr discs
KBr is used in FTIR for preparing solid samples. Where the solid is grinded together with KBr and compressed together to form a transparent disc. KBr is an IR-transparent material that does not absorb infrared light in the region typically used for analysis, allowing the sample’s spectrum to be observed without interference.
Infrared light from the FTIR instrument passes through the KBr disc. Because KBr is transparent to IR light, the light interacts primarily with the sample embedded within the disc.
The sample absorbs specific wavelengths of the IR light, corresponding to the vibrational modes of its molecular bonds. The FTIR instrument measures the intensity of the transmitted light and generates a spectrum, which is a plot of absorbance (or transmittance) wavenumber.
The resulting spectrum is analysed to identify functional groups and molecular structures based on characteristic absorption peaks.
Salt discs
Salt discs are typically made from sodium chloride (NaCl) or potassium bromide (KBr), are commonly used in FTIR spectroscopy for the preparation and analysis of liquid and solid samples. These discs are IR-transparent and allow for the measurement of samples without interference from the disc material itself.
Sodium chloride (NaCl) and potassium bromide (KBr) are the most common salts used. NaCl is usually preferred for liquids due to its excellent IR transparency from 4000 to 625 cm⁻.
The liquid sample is sandwiched between two discs. Infrared light passes through the salt disc and the liquid sample. The salt discs allow IR light to pass through without absorbing it, except at very low or very high wavelengths outside the typical IR range.
The FTIR spectrometer measures the intensity of IR light before and after passing through the sample, generating an absorbance or transmittance spectrum.
Sources
How an FTIR Spectrometer Operates – Chemistry LibreTexts
How Does FTIR Analysis Work? | Innovatech Labs
Guide to FT-IR Spectroscopy | Bruker
Fourier Transform Infrared Spectroscopy – an overview | ScienceDirect Topics
FTIR: Transmission vs ATR spectroscopy | Animated Guides – Specac Ltd
How is Potassium Bromide Used in Infrared Spectroscopy? (azom.com)
