If you find this useful, please leave a comment at the end of the page.
A link to a video of this paper can be found in the link: Master OCR 2022 AS Chemistry Paper 2 (H032/02) | Step-by-Step Solutions

Answer all the questions
1
Lime is a citrus fruit containing citric acid, C6H8O7.
(a)
Citric acid is a weak organic acid.
(i)
What is meant by an acid?
Acid is a substance that provides ions H+
[1]
(ii)
What is meant by an acid that is weak?
Weak acid is an acid that partially dissociates
[1]
(b)
A student carries out a titration to determine the mass of citric acid in a lime.
The student follows the method below:
- Squeeze the juice out of two limes.
- Transfer the juice into a 250.0 cm3 volumetric flask and make up to the mark with distilled water.
- Pipette 25.0 cm3 of the diluted lime juice into a conical flask and add a few drops of phenolphthalein indicator.
- Titrate this solution with 0.800 mol dm–3 NaOH(aq).
The student carries out a trial titration, followed by three further titrations.
The diagram shows the burette readings for the three further titrations.
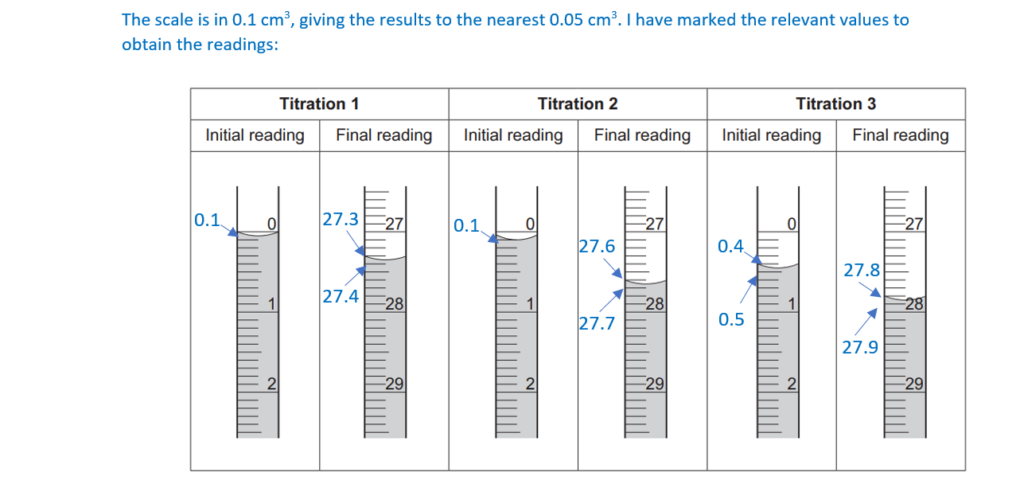
Each reading is measured to the nearest 0.05 cm3.
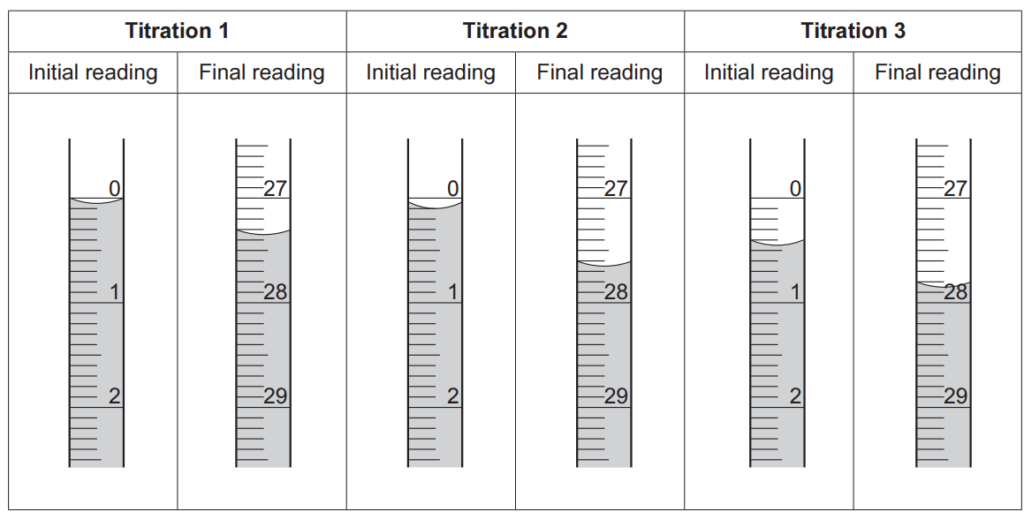
(i)
Record the student’s burette readings in the table below.
Calculate the mean titre, to the nearest 0.05 cm3, that the student should use to analyse the results.
mean titre ………………………………………….. cm3 [4]
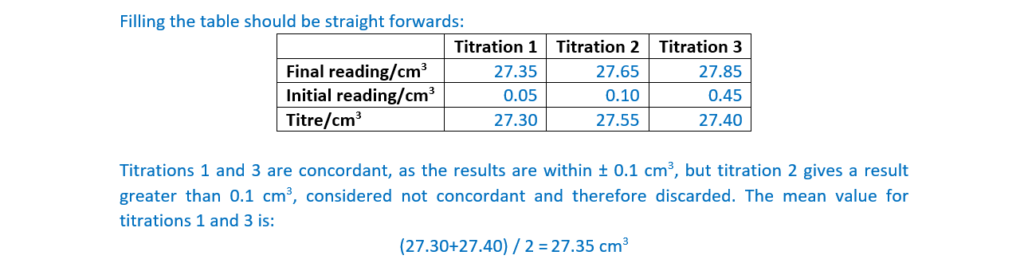
mean titre …………………27.35……………………….. cm3 [4]
(ii)
Citric acid, C6H8O7, is neutralised by NaOH as shown in the equation below.
C6H8O7 + 3NaOH → Na3C6H5O7 + 3H2O
Calculate the mass, in g, of citric acid in one lime.
Assume that citric acid (Mr = 192.0) is the only acid in lime juice.
mass of citric acid in one lime = ……………………………………………… g [5]

(c)
The student’s teacher thinks that there is an unnecessary safety risk in using a sodium hydroxide concentration of 0.800 mol dm–3 for the titration.
Suggest how the student could modify the method using a sodium hydroxide concentration of 0.200 mol dm–3 instead of 0.800 mol dm–3.
The student should aim to have the same titre as in the original method.
Justify your answer.
[2]

(d)
Other fruits contain different organic acids.
Apple juice contains malic acid which has the following structure.
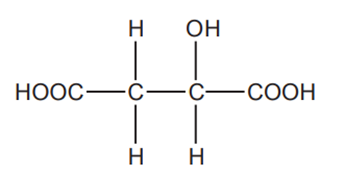
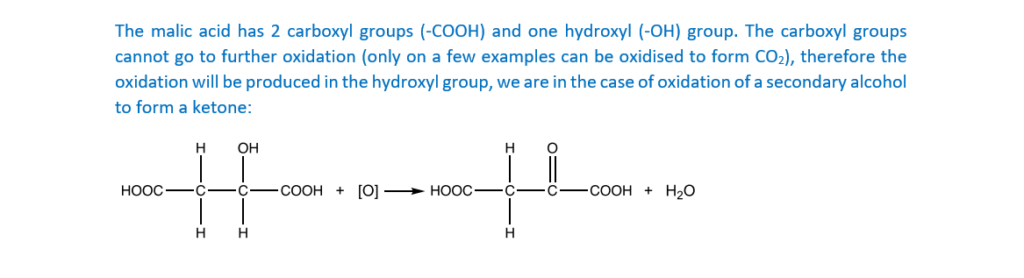
Malic acid can be oxidised by heating with acidified potassium dichromate(VI).
Write a balanced equation for the reaction, showing the structure of the organic product.
Use [O] to represent the oxidising agent.
[2]
2
This question is about some Group 2 elements and their compounds.
(a)
Strontium and calcium both react with water.
(i)
Write an equation for the reaction of strontium with water.
…………………………………………………………………………………………………………………….. [1]

(ii)
Using oxidation numbers, explain why the reaction of strontium with water is a redox reaction.
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
……………………………………………………………………………………………………………………..
[2]

(iii)
Explain why calcium reacts more slowly with water than strontium does.
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
……………………………………………………………………………………………………………………..
[3]

(b)
A student adds barium oxide, BaO, to water.
A reaction takes place forming a colourless solution.
(i)
Predict the approximate pH of the colourless solution.
pH = …………….
[1]

(ii)
A student adds a few drops of dilute sulfuric acid to the colourless solution.
Describe what the student would observe, and give the formula of the barium compound produced.
Observation ………………………………………………………………………………………………………..
Formula of barium compound ……………………………………………………………………………….
[2]

3
A student investigates some reactions of zinc compounds and zinc metal.
(a)
The student investigates the rate of reaction between zinc carbonate, ZnCO3(s), and dilute hydrochloric acid, HCl(aq).
ZnCO3(s) + 2HCl(aq) → ZnCl2(aq) + CO2(g) + H2O(l)
The student follows the method outlined below:
- Add 50 cm3 of dilute HCl(aq) into a conical flask at 20°C.
- Place the flask on a top-pan balance.
- Add an excess of ZnCO3(s) to the flask.
- Record the mass of the flask and contents on the top-pan balance every 30 seconds.
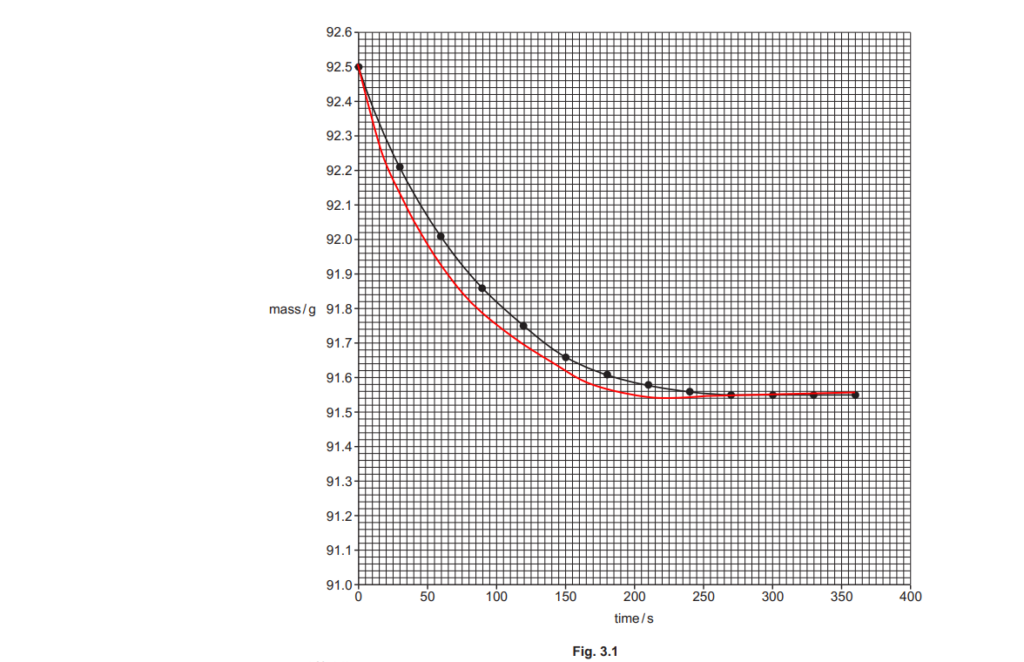
The student plots a graph of mass against time, shown in Fig. 3.1 below.
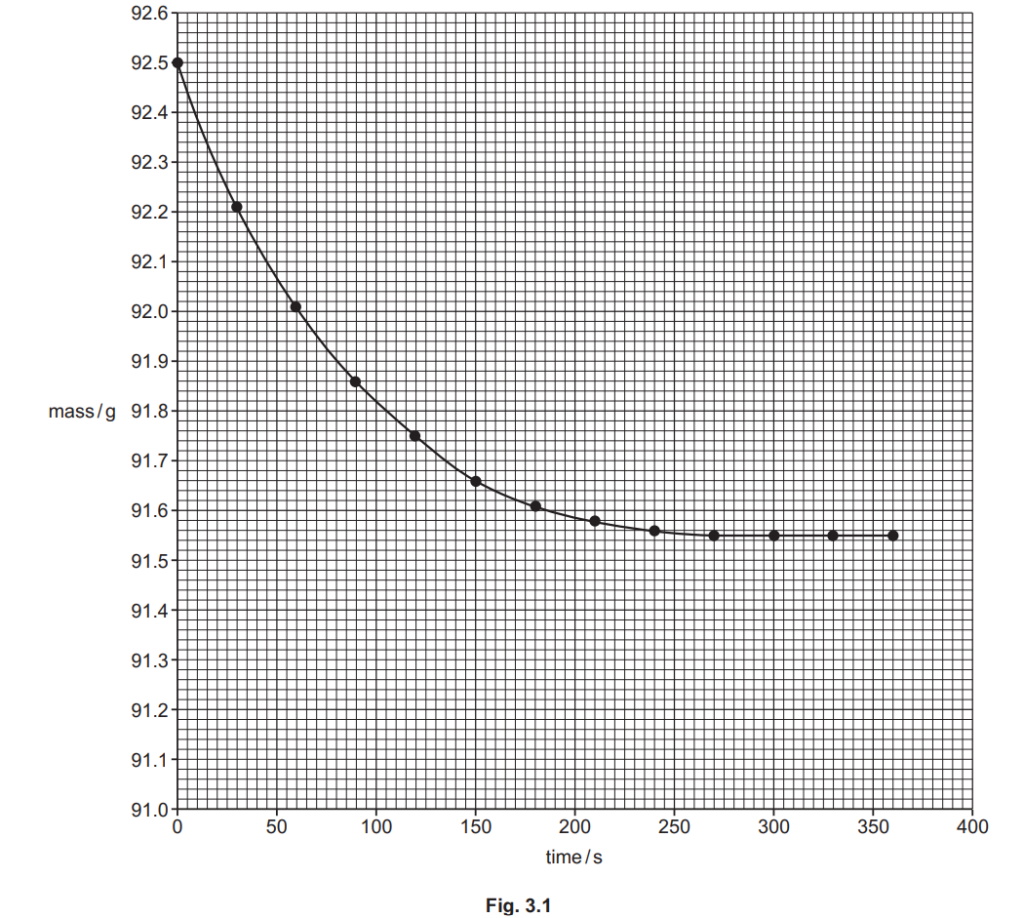
(i)
The graph shows that the reaction gets slower over time, and eventually stops.
Explain why, in terms of collision theory.
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
……………………………………………………………………………………………………………………..
[3]

(ii)
Using the graph in Fig. 3.1, find the rate of reaction, in g s–1, at 50 seconds.
Show your working on the graph and in the space below.
rate of reaction = …………………………………………. g s–1 [2]

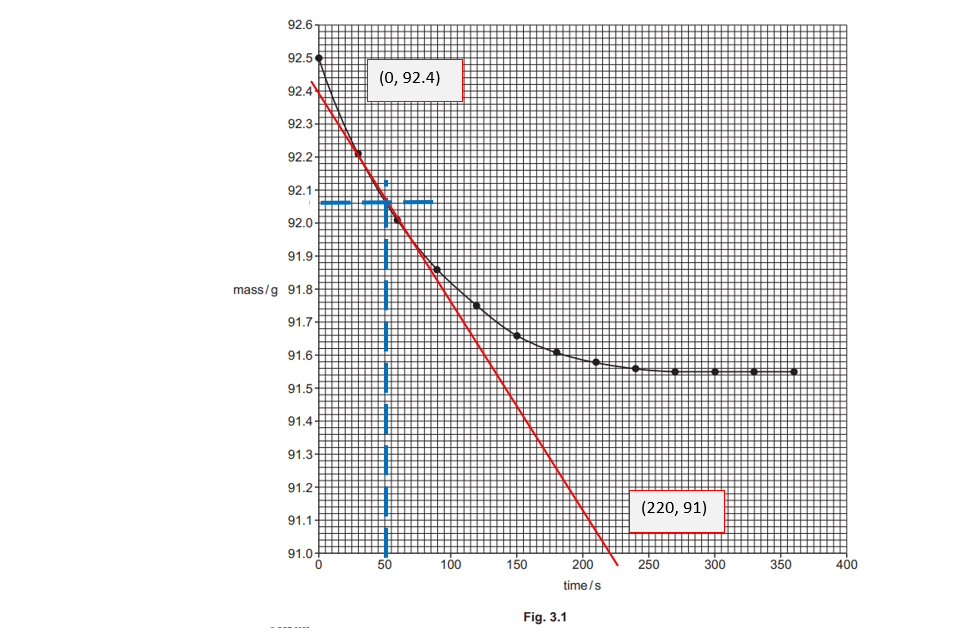

(iii)
The student repeats the experiment but heats 50 cm3 of dilute hydrochloric acid up to 40°C before adding the ZnCO3(s).
On Fig. 3.1, sketch the curve the student would obtain.
[2]

(b)
The student investigates the reaction between zinc and dilute sulfuric acid.
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g) ΔH = –140kJ mol–1
Copper(II) sulfate is a catalyst for this reaction.
- The student adds a piece of zinc to each of two test tubes.
- The student adds a few drops of aqueous copper(II) sulfate to one of the test tubes, forming a pale blue solution.
- The student adds an excess of dilute sulfuric acid to each test tube.
(i)
Describe two differences the student would observe between the test tubes.
1 ……………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
2 ……………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
[2]

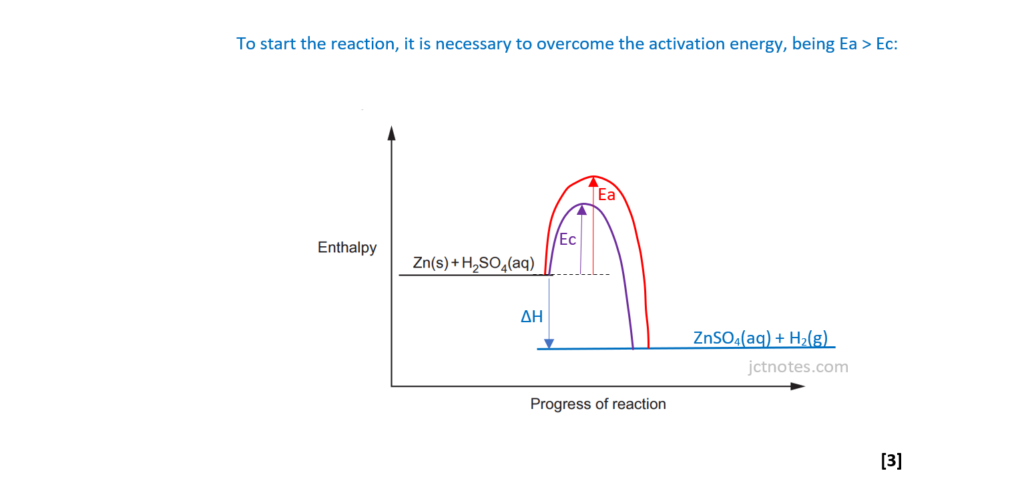
(ii)
Using the axes below, sketch an enthalpy profile diagram for the reaction with and without the catalyst.
On your diagram, include the following labels:
- ΔH, the enthalpy change
- Ea, the activation energy without a catalyst
- Ec, the activation energy with a catalyst.
4
This question is about the manufacture of hydrogen, H2.
(a)
In industry, hydrogen is manufactured from methane, as shown in Equilibrium 4.1.
CH4(g) + H2O(g) → CO(g) + 3H2(g) ΔH = +206 kJ mol–1 Equilibrium 4.1
The industrial process is carried out at 15 atmospheres pressure and at a temperature of 800°C using an excess of steam. A nickel catalyst is used
(i)*
Explain why these conditions are used industrially.
[6]

(ii)
A chemist mixes CH4(g) and H2O(g) and leaves the mixture to reach equilibrium.
CH4(g) + H2O(g) → CO(g) + 3H2(g) ΔH = +206 kJ mol–1 Equilibrium 4.1
The equilibrium mixture contains the following concentrations.
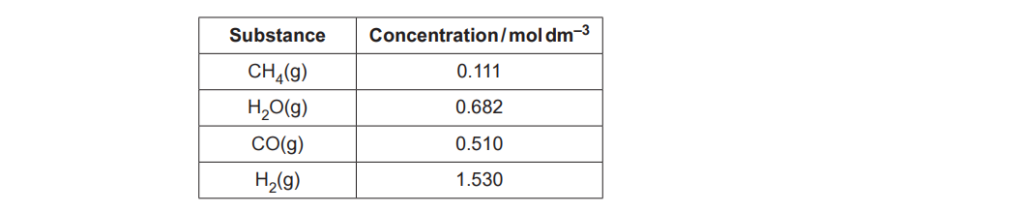
Write an expression for the equilibrium constant, Kc, for Equilibrium 4.1 and calculate the numerical value of Kc.
Give your answer to 3 significant figures.
Kc = ………………………………….. mol2 dm–6 [2]
If you want more information about equilibrium constant, check the link Chemical Equilibrium – (jctnotes.com)
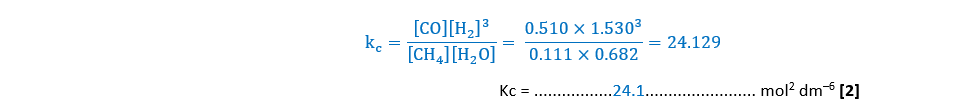
(b)
Hydrogen can also be manufactured by reacting ethanol with steam, as shown in Equilibrium 4.2.
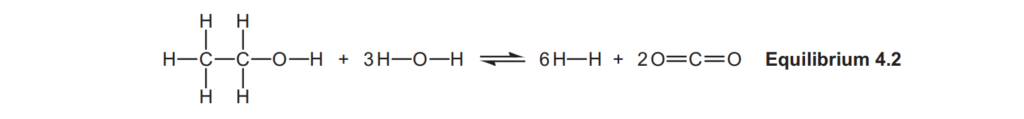
Average bond enthalpies are shown in the table below.
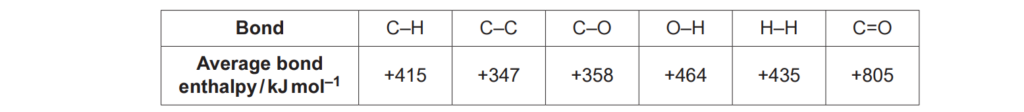
Calculate ΔH, in kJ mol–1, for the forward reaction in Equilibrium 4.2.
ΔH = …………………………………….. kJ mol–1 [3]



(c)
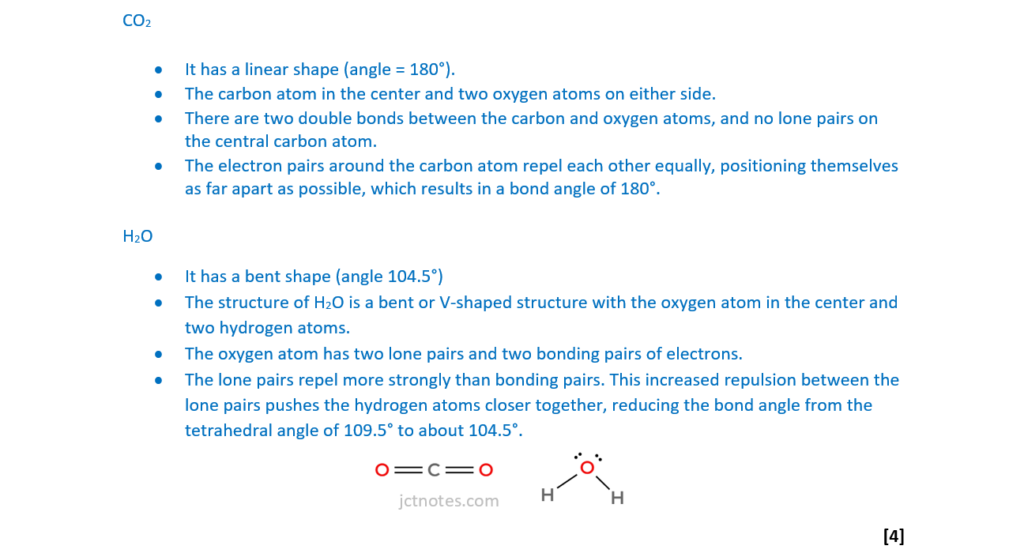
CO2 and H2O molecules have different shapes.
State the bond angles in CO2 and H2O molecules and explain, in terms of electron pair repulsion, why the bond angles are different.
[4]
5
2-Bromobutane, CH3CH2CHBrCH3, can be prepared by three different methods.
The relative molecular mass, Mr, of 2-bromobutane is 136.9
(a)
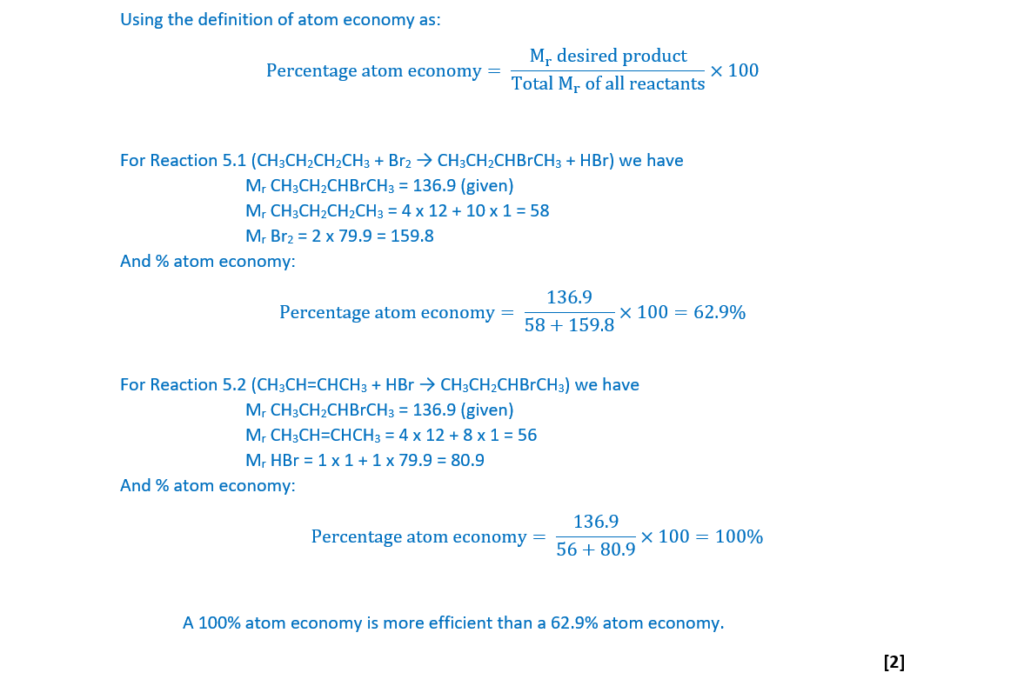
2-Bromobutane can be prepared by reacting butane with bromine (Reaction 5.1).
CH3CH2CH2CH3 + Br2 → CH3CH2CHBrCH3 + HBr Reaction 5.1
The reaction is initiated by the formation of bromine radicals from bromine.
(i)
State the conditions for the formation of bromine radicals from bromine.
……Bromine radicals are produced by UV light.………………………………………………………………………………………………….. [1]
(ii)
Write two equations for the propagation steps in the mechanism for Reaction 5.1.
Use structural formulae for organic species and dots (•) for unpaired electrons on radicals.
CH3CH2CH2CH3 + Br• → CH3CH2CH•CH3 + HBr
CH3CH2CH•CH3 + Br2 → CH3CH2CHBrCH3 + Br•
[2]
(b)
2-Bromobutane can also be prepared by reacting but-2-ene, CH3CH=CHCH3, with hydrogen bromide, HBr (Reaction 5.2).
CH3CH=CHCH3 + HBr → CH3CH2CHBrCH3 Reaction 5.2
Explain, in terms of atom economy, why Reaction 5.2 is more sustainable than Reaction 5.1.
Include calculations to justify your answer.
[2]
(c)
2-Bromobutane can also be prepared by reacting butan-2-ol, CH3CH2CHOHCH3, with sodium bromide and sulfuric acid (Reaction 5.3).
CH3CH2CHOHCH3 + H+ + Br– → CH3CH2CHBrCH3 + H2O Reaction 5.3
2-Bromobutane is a liquid with a boiling point of 91°C and does not mix with water.
(i)
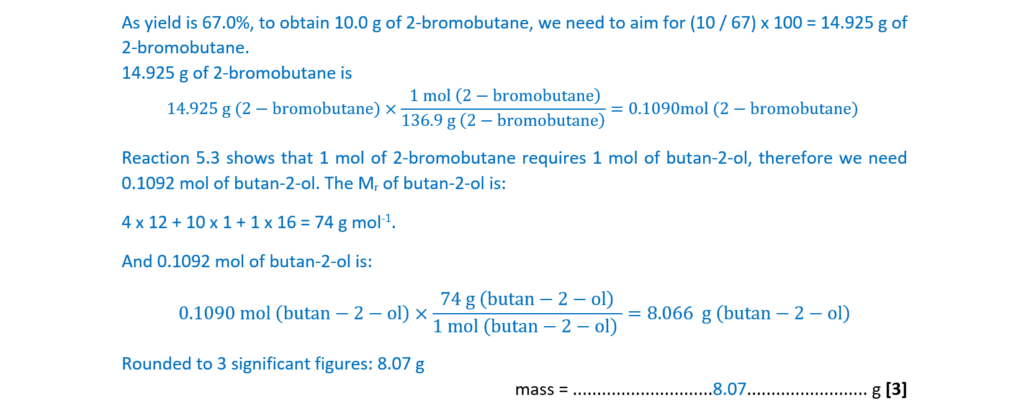
A student plans to prepare 10.0 g of 2-bromobutane using Reaction 5.3.
The percentage yield is 67.0%.
Calculate the mass of CH3CH2CHOHCH3 needed for this preparation.
Give your answer to 3 significant figures.
mass = ……………………………………………… g [3]
(ii)
The student mixes butan-2-ol, sodium bromide and sulfuric acid in a pear-shaped flask, and refluxes the mixture.
After 1 hour, the mixture in the flask has separated into two layers: an aqueous layer and an organic layer.
Describe the procedures the student would need to carry out to obtain a pure, dry sample of 2-bromobutane from this mixture.
[3]
1st separate from the mixture the organic layer from the aqueous layer using a separating funnel. Note: as we don’t know the densities, we cannot say which layer will be on top and which on bottom, so don’t specify.
2nd dry the organic layer with an anhydrous salt (such as CaCl2, Na2SO4, MgSO4 or CaSO4).
3rd distil and collect the fraction at 91°C (boiling point of 2-Bromobutane).
. [3]
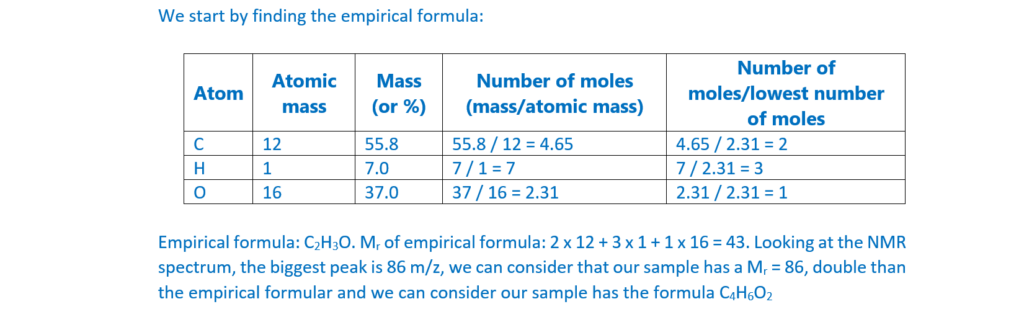
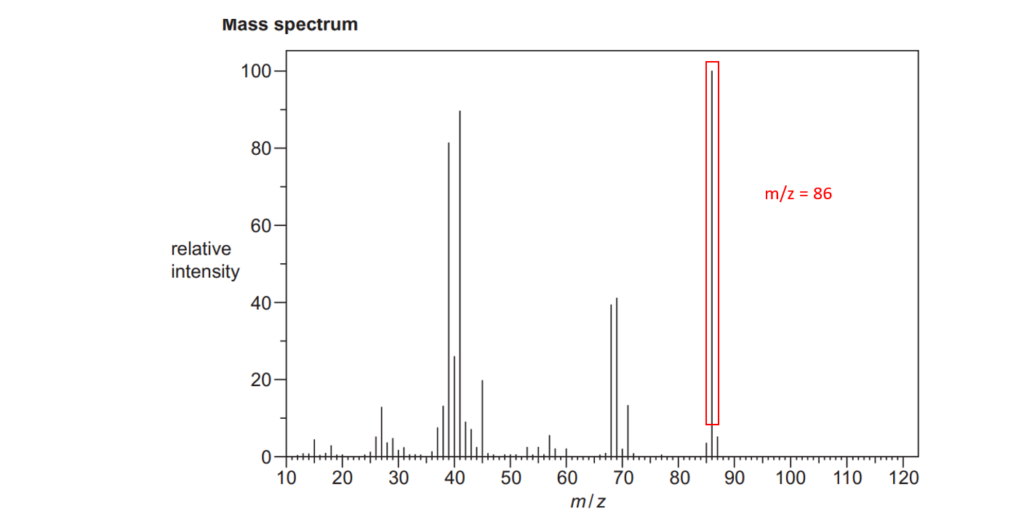
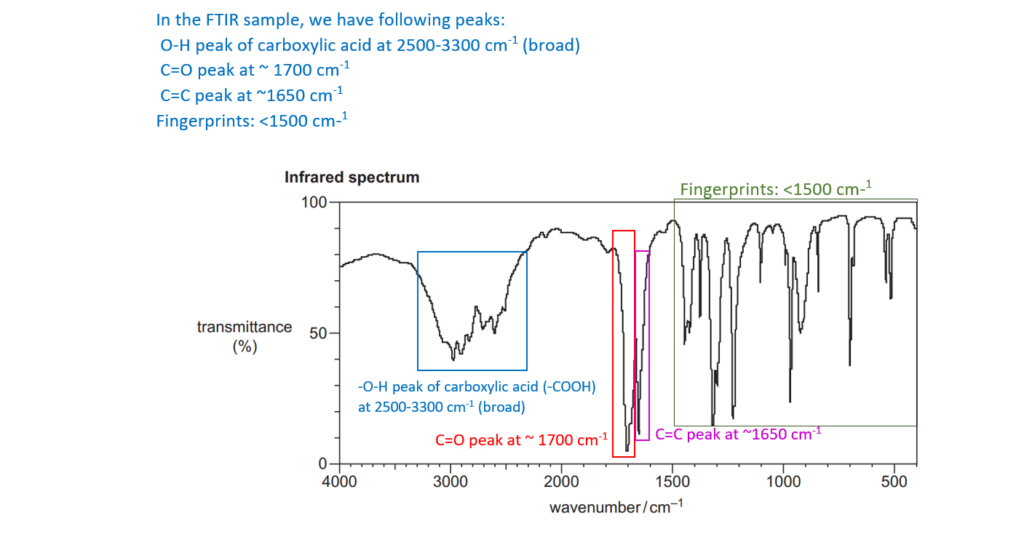
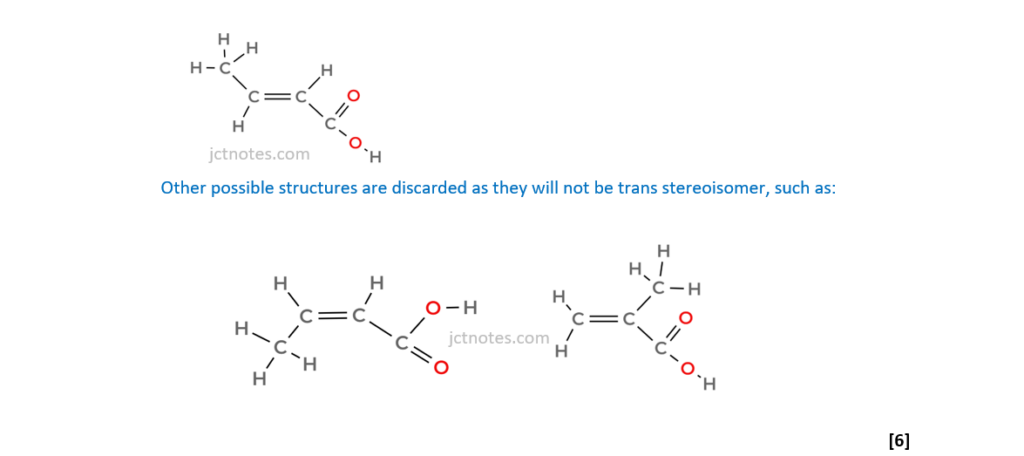
6*
The organic compound A is unsaturated and is a trans stereoisomer.
Compound A has the following composition by mass: C, 55.8%; H, 7.0%; O, 37.2%.
The mass spectrum and the infrared spectrum of compound A are shown below.
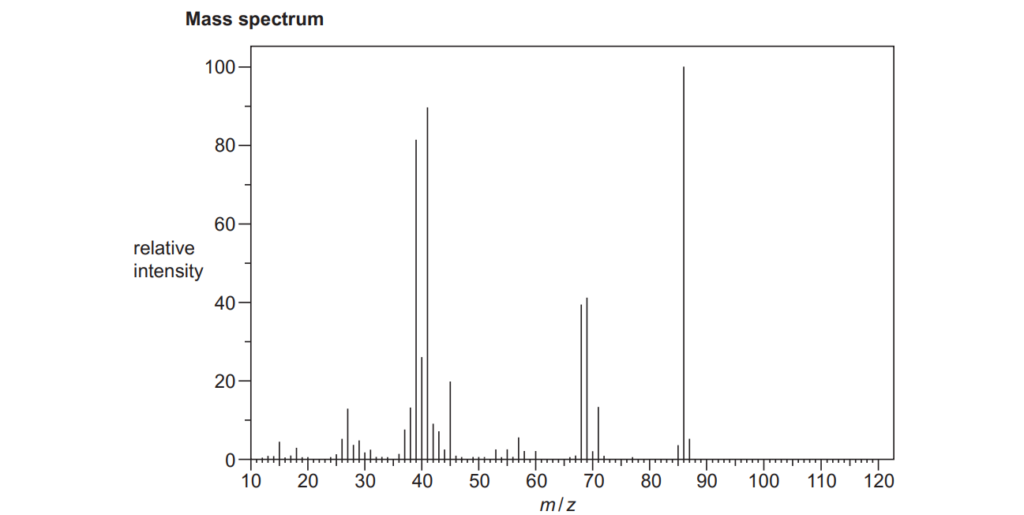
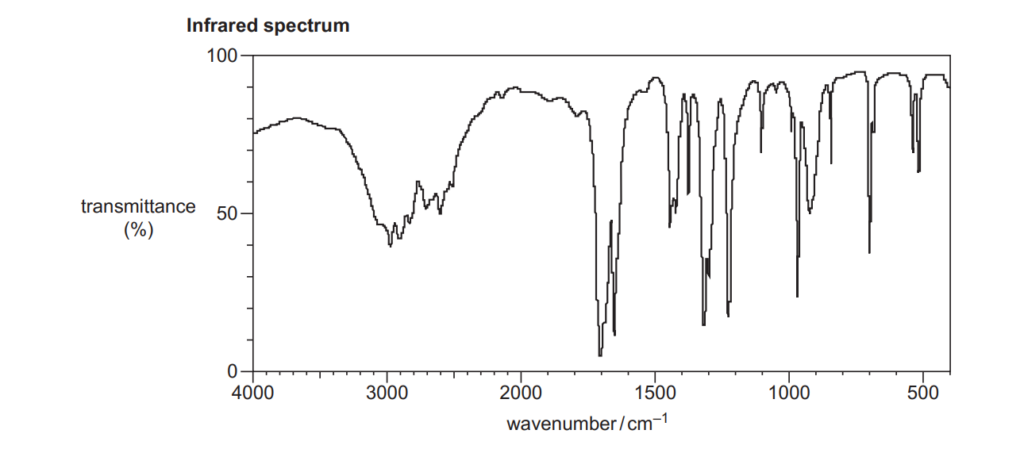
Use the information to determine the structure of compound A.
Explain your reasoning and show your working.
[6]
For an explanation about how to calculate empirical formulas, see the following link: Formulas and formula masses – (jctnotes.com)
END OF QUESTION PAPER
External links:
- Paper: AS Level Chemistry A H032/02 Depth in chemistry June 2022 (ocr.org.uk)
- Mark scheme: Mark Scheme H032/02 Depth in chemistry June 2022 (ocr.org.uk)
- OCR data sheet for the exam: AS GCE (H032) A GCE (H432) Data Sheet for Chemistry A (ocr.org.uk)
