On this page, I explain the basic of balancing chemical equations. I assume that you understand the basic of chemical formulas and moles, I explain these concepts in another page. If you are looking how to balance redox equations, click in this link.
The outline for this page is:
Theory
I have created a video in YouTube based on the theory explained here. You can see the video by clicking here.
A chemical equation is an equation used to describe a chemical reaction, stating the chemical species involved and their proportions. For example: for the reaction
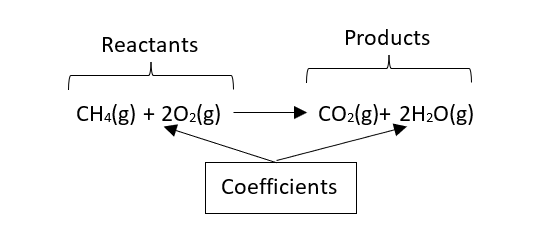
Methane + Oxygen → Carbon dioxide + water
The equation is
CH4(g) + 2O2(g) → CO2(g)+ 2H2O(g)
The equation indicates that 1 molecule (or mol) of methane gas reacts with 2 molecules (or moles) of oxygen gas to give 1 molecule (or mol) of carbon dioxide gas and 2 molecules (or moles) of water gas (gas at room temperature is a liquid, however, as this reaction is exothermic and generate a considerable amount of heat, the water appears as vapour). Sometimes is required to write in brackets the physical state of the molecules, the state symbols used are:
| State symbol | Meaning |
| (s) | Solid |
| (l) | Liquid |
| (g) | Gas |
| (aq) | Aqueous solution |
The compounds on the left are called reactants and on the right products. The number that indicates the number of molecules or mols are called coefficients. In the examples below, the reactants are CH4 and O2, the products: CO2 and H2O, the coefficients of the O2 and H2O is 2 and the coefficient of the CH4 and CO2 is 1 (when it is 1, it is usually omitted):
Balancing chemical equations is essential for understanding and predicting the behavior of chemical reactions, and for making quantitative predictions about the amounts of reactants and products involved in a reaction.
I will explain two methods to balance equations: the traditional method (it is easier for most of the cases and it helps to improve your knowledge of chemistry) and the algebraic method (based on solving mathematical equations, I use this method when the traditional method is challenging).
Traditional method
Rules:
- Write down each formula of each substance involved in the equation.
- Bear in mind that the final number of each atom in the reactants and in the products should be the same. If you want to sound more technical, you can say that you are applying the law of the conservation of mass.
- Identify the most complex substance. Try to find an atom (element) or ionic component (like SO42-, NO3–, …) that appears in only one reactant and in only one product, if possible, and adjust the coefficients to ensure that the same number of atoms are on both sides of the equation.
- Continue balancing the equation, leaving the simple atoms at the end.
- Coefficients can be written as simple fractions (1/2, 2/3, …), if you don’t like fractions, we can multiply the whole equation (i.e. every single molecule in the equation) for an adequate number to make them whole numbers, for example:
Na(s) + H2O → NaOH(aq) + ½ H2(g)
can also be written as
2Na(s) + 2H2O → 2NaOH(aq) + H2(g)
EXAMPLE:
Given the equation
CH4(g) + O2(g) → CO2(g)+ H2O(g)
I fix CH4 to 1
1CH4(g) + O2(g) → CO2(g)+ H2O(g)
As I start with 1 atom of C and 4 atoms of H (no other reactants have C or H), in the products I will finish with 1 atom of C and 4 atoms of H. Let’s go by steps:
1CH4(g) + O2(g) → 1CO2(g)+ H2O(g)
1CH4(g) + O2(g) → 1CO2(g)+ 2H2O(g)
The only element left is the O, and we have fixed the molecules of CH4, CO2 and H2O, so the only component remained to fix is the O2. Let’s work by steps:
1CH4(g) + O2(g) → 1CO2(g)+ 2H2O(g)
1CH4(g) + 2O2(g) → 1CO2(g)+ 2H2O(g)
Algebraic method
(Also known as Bottomley’s method). This method uses algebraic equations. Steps are:
- Write down each formula of each substance involved in the equation.
- Assign a letter for each coefficient in the chemical equation
- Group the letters by atoms or ionic species creating equations making those that appear as reactant in one side (say left) and those of the products on the other (say right)
- Assign an arbitrary number (typically 1) to a coefficient (letter)
- Resolve the equations
Given the equation
CH4(g) + O2(g) → CO2(g)+ H2O(g)
I assign a letter to each coefficient
aCH4(g) + bO2(g) → cCO2(g)+ dH2O(g)
I group the letters by elements and creating equations, bearing in mind that the numbers on one side are for the reactants on the other those for products:
- C: as reactant appears once as a (from the CH4) and as product appear once as c (from CO2), i.e.:
C: a = c
- H: as reactant appears 4 times as a (from the CH4) and as product appears 2 times as d (from H2O), i.e.:
H: 4a = 2d
- O: as reactant appears twice b (from the O2) and as product appears twice as c (from CO2) and once as d (1 time from H2O), i.e.:
O: 2b = 2c + d
Putting all together:
C: a = c
H: 4a = 2d
O: 2b = 2c + d
Now, I fix 1 letter, say a = 1. If a = 1:
(a =) c => 1 = c
4a = 2d => 4×1 = 2d => d = 4/2 = 2
2b = 2c + d => 2b = 2×1 + 2 = 2 +2 = 4 => b = 4/2 = 2
Replacing a, b, c and d in the original equation:
CH4(g) + 2O2(g) → CO2(g)+ 2H2O(g)
To ensure that the equation is balanced, we need to check that the number of atoms that appear in the reactants are the same that in the products
| Atom | Number in reactants | Number in products |
| C | 1 from CH4 Total: 1 | 1 from CO2 Total: 1 |
| H | 4 from CH4 Total: 4 | 2 from each H2O: i.e.: 4 Total: 4 |
| O | 2 from each O2 Total: 4 | 2 from each CO2, i.e.: 2 1 from each H2O: i.e.: 2 Total: 4 |
===================================================================
Explained exercises
1. TiCl4 + Mg → Ti + MgCl2
Answer: TiCl4 + 2Mg → Ti + 2MgCl2
Steps (traditional):
i. Fixing TiCl4 in reactants to 1:
1TiCl4 + Mg → Ti + MgCl2
ii. Adjusting the products to 1 Ti and 4 Cl:
(1)TiCl4 + Mg → 1Ti + 2MgCl2
iii. The only element left is Mg from the reactants, as we have 2 Mg from the products, this should be 2 in the reactants:
(1)TiCl4 + 2Mg → (1)Ti + 2MgCl2
i.e: TiCl4 + 2Mg → Ti + 2MgCl2
Steps (algebra):
aTiCl4 + bMg → cTi + dMgCl2
Algebraic equations:
Ti: a = c
Cl: 4a = 2d
Mg: b = d
Resolution I fix a = 1, then:
a = 1
c = 1
4a = 2d => 4 = 2d => d = 2
b = d => b = 2
Result: TiCl4 + 2Mg → Ti + 2MgCl2
| Atom | Number in reactants | Number in products |
| Ti | 1 | 1 |
| Cl | 4 | 2 x 2 = 4 |
| Mg | 2 | 2 |
2. Na(s) + H2O → NaOH(aq) + H2(g)
Answer: Na(s) + H2O → NaOH(aq) + 1/2H2(g)
Or: 2Na(s) + 2H2O → 2NaOH(aq) + H2(g)
Steps (traditional):
i. Fixing Na in reactants to 1:
1Na(s) + H2O → NaOH(aq) + H2(g)
ii. In products, Na only appears in NaOH, then, we fix it to 1
(1)Na(s) + H2O → (1)NaOH(aq) + H2(g)
iii. Inspecting what we have so far, we can see that we have left are the atoms of O and the H:
– the O appears in H2O (not fixed yet) and NaOH (fixed)
– the H appears in H2O (not fixed yet), NaOH (fixed) and H2 (not fixed yet)
therefore, it is easier to use the O and fix the H2O (if we use the H, we will have 2 to fix: H2O and H2). As in the products we have fixed the atoms to O to 1, in the reactants we need to fix it to 1, i.e. the H2O is 1:
(1)Na(s) + (1)H2O → (1)NaOH(aq) + H2(g)
iv. The only element left is the H. We have 2 atoms of H in the reactants via H2O (already fixed) and in the products we have 1 atom of H in the NaOH (already fixed) and 2 atoms of H in the H2 (not fixed yet). To make ensure that the number of atoms of H in both sides of the equation are equal, each molecule (mol) of H2 should contribute with 1 atom, so we need ½ molecule (mol) of H2.
(1)Na(s) + (1)H2O → (1)NaOH(aq) + (1/2)H2(g)
v. This is the answer, however, if you don’t want fraction, multiply the whole equation (reactants and products) by 2
2Na(s) + 2H2O → 2NaOH(aq) + H2(g)
Steps (Algebra):
i) Inserting the coefficients: aNa(s) + bH2O → cNaOH(aq) + dH2(g)
ii) Write the equations:
Na: a = c
H: 2b = c + 2d
O: b = c
iii) Fix 1 coefficient to 1, say a = 1
iv) Solve the system
If a = 1, then:
As a = c => c = 1
As b = c => b = 1
We only have d to solve: 2b = c + 2d => 2×1 = 1 + 2xd => d = ½
v) Write the result:
Na(s) + H2O → NaOH(aq) + 1/2H2(g)
Or: 2Na(s) + 2H2O → 2NaOH(aq) + H2(g)
Counting the atoms (the 1st number is for the 1st result given, the number in brackets is the number in the 2nd results given)
| Atom | Number in reactants | Number in products |
| Na | 1(2) | 1(2) |
| H | 2(4) | 2(4) |
| O | 1(2) | 1(2) |
3. CH2OH + O2 → CO2 + H2O
Answer: 4CH2OH + 5O2 → 4CO2 + 6H2O
Steps (traditional):
i. I fix the CH2OH to 1,
1CH2OH + O2 → CO2 + 3H2O
ii. and this fix the number of the atoms of C and H on the products (C and H are only provided by the CH2OH, but the atom of O is also given by O2). Let’s do by steps:
a) Atoms of C on the products: I only have 1 atom of C from the reactants, then I can only have 1 atom of C on the products, and therefore I only have 1 CO2:
1CH2OH + O2 → 1CO2 + H2O
b) Atoms of H on the products: I only have 3 atoms of H from the reactants, then I can only have 3 atoms of H on the products, all of them in the molecule of H2O. As each molecule of H2O has 2 atoms of H, I need 3/2 molecules of H2O:
1CH2OH + O2 → 1CO2 + 3/2H2O
iii. The only atom left is the O and the molecule of O2 in the reactants.
a) In the products, I have 2 atoms of O from the molecule of CO2 and 3/2 of atoms from the H2O, giving me a total of 2 + 3/2 = 7/2
b) In the reactants I have 1 atom of O from the CH2OH, the ((7/2)-1 = 5/2) needed has to come from the O2, as each molecule of O2 contribute with 2 atoms of O, the number has to be divided by 2 ((5/2)/2 = 5/4)
1CH2OH + 5/4O2 → 1CO2 + 3/2H2O
iv. The equation is solved, to remove the fraction, you can multiply it by 4:
4CH2OH + 5O2 → 4CO2 + 6H2O
Steps (algebra):
i) Inset coefficients
aCH2OH + bO2 → cCO2 + dH2O
ii) Equations:
C: a = c
H: 3a = 2d
O: a + 2b = 2c + d
iii) Fixing 1 coefficient, say a = 1
iv) Solve the system
As a = c => c = 1
As 3a = 2d => 3×1 = 2d => d = 3/2
As a + 2b = 2c + d => 1 + 2b = 2×1 + 3/2 => b = 5/4
v) Write the equation replacing the coefficients by their values:
CH2OH + 5/4O2 → CO2 + 3/2H2O
Or multiplying by 4:
4CH2OH + 5O2 → 4CO2 + 6H2O
| Atom | Number in reactants | Number in products |
| C | 4 | 4 |
| H | 12 | 12 |
| O | 14 | 14 |
Exercises
List of exercises. Click in the number of the exercise to go to the exercise.
| Number | Question | Answer | Level |
| 1 | Mg + O2 → MgO | 2Mg + O2 → 2MgO | Easy |
| 2 | Fe + Cl2→ FeCl3 | 2Fe + 3Cl2 → 2 FeCl3 | Easy |
| 3 | Ca + H20 → Ca(OH)2 + H2 | Ca + 2 H20 → Ca(OH)2 + H2 | Easy |
| 4 | C7H16 + O2 → CO2 + H2O | C7H16 + 11O2 → 7CO2 + 8H2O | Easy |
| 5 | C8H18 + O2 → CO2 + H2O | 2C8H18 + 25O2 → 16CO2 + 18H2O | Easy |
| 6 | PCl5 + H2O → H3PO4 + HCl | PCl5 + 4H2O → H3PO4 + 5HCl | Medium |
| 7 | P4O10 + H2O → H3PO42 | P4O10 + 6H2O → 4H3PO4 | Medium |
| 8 | SiCl4 + H2O → H4SiO4 + HCl | SiCl4 + 4H2O → H4SiO4 + 4HCl | Medium |
| 9 | Al + HCl → AlCl3 + H2 | 2Al + 6HCl → 2AlCl3 + 3H2 | Easy |
| 10 | Na2CO3 + HCl → NaCl + H2O + CO2 | Na2CO3 + 2HCl → 2NaCl + H2O + CO2 | Hard |
| 11 | Fe2(SO4)3 + KOH → K2SO4 + Fe(OH)3 | Fe2(SO4)3 + 6KOH → 3K2SO4 + 2Fe(OH)3 | Medium |
| 12 | Ca3(PO4)2 + SiO2 → P4O10 + CaSiO3 | 2Ca3(PO4)2 + 6SiO2 → P4O10 + 6CaSiO3 | Hard |
| 13 | KClO3 → KClO4 + KCl | 4KClO3 → 3KClO4 + KCl | Hard |
| 14 | Al2(SO4)3 + Ca(OH)2 → Al(OH)3 + CaSO4 | Al2(SO4)3 + 3Ca(OH)2 → 2Al(OH)3 + 3CaSO4 | Medium |
| 15 | H2SO4 + HI → H2S + I2 + H2O | H2SO4 + 8HI → H2S + 4I2 + 4H2O | Hard |
| 16 | Al + O2 → Al2O3 | 4Al + 3O2 → 2Al2O3 | Medium |
| 17 | Al(NO3)3 + NaOH → Al(OH)3 + NaNO3 | Al(NO3)3 + 3NaOH → Al(OH)3 + 3NaNO3 | Medium |
| 18 | O2 + CS2 →CO2 + SO2 | 3O2 + CS2 →CO2 + 2SO2 | Medium |
| 19 | BaF2 + K3PO4 → Ba3(PO4)2 + KF | 3BaF2 + 2K3PO4 → Ba3(PO4)2 + 6KF | Medium |
| 20 | H2SO4 +Mg(NO3)2→ MgSO4 + HNO3 | H2SO4 +Mg(NO3)2→ MgSO4 + 2HNO3 |
1. Mg + O2 → MgO
Answer: 2Mg + O2 → 2MgO
Steps:
1Mg + O2 → MgO
1Mg + O2 → 1MgO
Mg + ½ O2 → MgO
2Mg + O2 → 2 MgO
| Atom | Number in reactants | Number in products |
| Mg | 2 | 2 |
| O | 2 | 2 |
————————————————————
2. Fe + Cl2→ FeCl3
Answer: 2Fe + 3Cl2 →2FeCl3
Steps:
Fe + Cl2 → 2FeCl3
Fe + 3Cl2→ 2FeCl3
2Fe + 3Cl2 → 2FeCl3
| Atom | Number in reactants | Number in products |
| Fe | 2 | 2 |
| Cl | 6 | 6 |
————————————————————
3. Ca + H20 → Ca(OH)2 + H2
Answer: Ca + 2 H20 → Ca(OH)2 + H2
Steps:
1Ca + H20 → Ca(OH)2 + H2
1Ca + H20 → 1Ca(OH)2 + H2
1Ca + 2H20 → 1Ca(OH)2 + H2
1Ca + 2H20 → 1Ca(OH)2 + 1H2
Ca + 2H20 → Ca(OH)2 + H2
| Atom | Number in reactants | Number in products |
| Ca | 1 | 1 |
| H | 4 | 4 |
| O | 2 | 2 |
————————————————————
4. C7H16 + O2 → CO2 + H2O
Answer: C7H16 + 11O2 → 7CO2 + 8H2O
Steps:
1C7H16 + O2 → CO2 + H2O
1C7H16 + O2 → 7CO2 + H2O
1C7H16 + O2 → 7CO2 + 8H2O
C7H16 + 11O2 → 7CO2 + 8H2O
C7H16 + 11O2 → 7CO2 + 8H2O
| Atoms | Number in reactants | Number in products |
| C | 7 | 7 |
| H | 16 | 16 |
| O | 22 | 22 |
————————————————————
5. C8H18 + O2 → CO2 + H2O
Answer: 2C8H18 + 25O2 → 16CO2 + 18H2O
Steps
1C8H18 + O2 → CO2 + H2O
1C8H18 + O2 → 8CO2 + H2O
1C8H18 + O2 → 8CO2 + 9H2O
1C8H18 + 25/2O2 → 8CO2 + 9H2O
1C8H18 + 25/2O2 → 8CO2 + 9H2O
2C8H18 + 25O2 → 16CO2 + 18H2O
| Atom | Number in reactants | Number in products |
| C | 16 | 16 |
| H | 32 | 32 |
| O | 50 | 50 |
————————————————————
6. PCl5 + H2O → H3PO4 + HCl
Answer: PCl5 + 4H2O → H3PO4 + 5HCl
Steps
1PCl5 + H2O → 1H3PO4 + HCl
1PCl5 + H2O → 1H3PO4 + HCl
1PCl5 + H2O → 1H3PO4 + 5HCl
1PCl5 + 4H2O → 1H3PO4 + 5HCl
1PCl5 + 4H2O → 1H3PO4 + 5HCl
PCl5 + 4H2O → H3PO4 + 5HCl
| Atom | Number in reactants | Number in products |
| P | 1 | 1 |
| Cl | 5 | 5 |
| H | 8 | 8 |
| O | 4 | 4 |
————————————————————
7. P4O10 + H2O → H3PO4
Answer: P4O10 + 6H2O → 4H3PO4
Steps:
1P4O10 + H2O → H3PO4
1P4O10 + H2O → 4H3PO4
1P4O10 + 6H2O → 4H3PO4
1P4O10 + 6H2O → 4H3PO4 (step not needed, just checking O atoms)
P4O10 + 6H2O → 4H3PO4
8. SiCl4 + H2O → H4SiO4 + HCl
Answer: SiCl4 + 4H2O → H4SiO4 + 4HCl
Steps:
1SiCl4 + H2O → 1H4SiO4 + HCl
1SiCl4 + H2O → 1H4SiO4 + HCl
1SiCl4 + H2O → 1H4SiO4 + 4HCl
1SiCl4 + 4H2O → 1H4SiO4 + 4HCl
1SiCl4 + 4H2O → 1H4SiO4 + 4HCl (step not needed, just checking H atoms)
SiCl4 + 4H2O → H4SiO4 + 4HCl
| Atom | Number in reactants | Number in products |
| Si | 1 | 1 |
| Cl | 4 | 4 |
| H | 8 | 8 |
| O | 4 | 4 |
9. Al + HCl → AlCl3 + H2
Answer: 2Al + 6HCl → 2AlCl3 + 3H2
Steps:
1Al + HCl → 1AlCl3 + H2
1Al + HCl → 1AlCl3 + H2
1Al + 3HCl → 1AlCl3 + H2
1Al + 3HCl → 1AlCl3 + 3/2H2
2Al + 6HCl → 2AlCl3 + 3H2
2Al + 6HCl → 2AlCl3 + 3H2
| Atom | Number in reactants | Number in products |
| Al | 2 | 2 |
| H | 6 | 6 |
| Cl | 6 | 6 |
————————————————————
10. Na2CO3 + HCl → NaCl + H2O + CO2
Answer: Na2CO3 + 2HCl → 2NaCl + H2O + CO2
Steps:
1Na2CO3 + HCl → 2NaCl + H2O + CO2
1Na2CO3 + HCl → 2NaCl + H2O + CO2
1Na2CO3 + HCl → 2NaCl + H2O + 1CO2
1Na2CO3 + HCl → 2NaCl + 1H2O + 1CO2
1Na2CO3 + 2HCl → 2NaCl + 1H2O + 1CO2
1Na2CO3 + 2HCl → 2NaCl + 1H2O + 1CO2 (step not needed, just checking Cl atoms)
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
| Atom | Number in reactants | Number in products |
| Na | 2 | 2 |
| C | 1 | 1 |
| O | 3 | 3 |
| H | 2 | 2 |
| Cl | 2 | 2 |
————————————————————
11. Fe2(SO4)3 + KOH → K2SO4 + Fe(OH)3
Answer: Fe2(SO4)3 + 6KOH → 3K2SO4 + 2Fe(OH)3
Steps
Note: SO42- and OH– are ionic components, treated as a whole unit.
1Fe2(SO4)3 + KOH → K2SO4 + Fe(OH)3
1Fe2(SO4)3 + KOH → K2SO4 + 2Fe(OH)3
1Fe2(SO4)3 + KOH → 3K2SO4 + 2Fe(OH)3
1Fe2(SO4)3 + 6KOH → 3K2SO4 + 2Fe(OH)3
1Fe2(SO4)3 + 6KOH → 3K2SO4 + 2Fe(OH)3 (step not needed, just checking OH–)
Fe2(SO4)3 + 6KOH → 3K2SO4 + 2Fe(OH)3
| Atom(s) | Number in reactants | Number in products |
| Fe | 2 | 2 |
| SO4 | 3 | 3 |
| K | 6 | 6 |
| OH | 6 | 6 |
————————————————————
12. Ca3(PO4)2 + SiO2 → P4O10 + CaSiO3
Answer: 2Ca3(PO4)2 + 6SiO2 → P4O10 + 6CaSiO3
Steps:
1Ca3(PO4)2 + SiO2 → P4O10 + CaSiO3
1Ca3(PO4)2 + SiO2 → P4O10 + 3CaSiO3
1Ca3(PO4)2 + SiO2 → 1/2P4O10 + 3CaSiO3
1Ca3(PO4)2 + 3SiO2 → 1/2P4O10 + 3CaSiO3
1Ca3(PO4)2 + 3SiO2 → 1/2P4O10 + 3CaSiO3 (step not needed, just checking O)
2Ca3(PO4)2 + 6SiO2 → 1P4O10 + 6CaSiO3 (adjusting coefficients)
2Ca3(PO4)2 + 6SiO2 → P4O10 + 6CaSiO3
| Atom | Number in reactants | Number in products |
| Ca | 6 | 6 |
| P | 4 | 4 |
| O | 28 | 28 |
| Si | 6 | 6 |
————————————————————
13. KClO3 → KClO4 + KCl
Answer: 4KClO3 → 3KClO4 + KCl
This problem is more complex that it seems at the beginning. As K and Cl appears in all the 3 molecules, we start with O, that only appears in 2 of the molecules and we make it equal in both sides of the equation:
4KClO3 → 3KClO4 + KCl
The only specie that we have left if KCl. We can start with Cl, or K, but as they are always in the same ratio (1:1), I put both of them together. If you prefer, you can be more systematic and do 1 by 1:
4KClO3 → 3KClO4 + 1KCl
4KClO3 → 3KClO4 + KCl
If you get stuck in a problem like this, it is worth to try the algebraic method:
aKClO3 → bKClO4 + cKCl
Equations:
K: a = b + c
Cl: a = b + c
O: 3a = 4b
Resolution:
Fix a = 1
From the O: 3×1 = 4b => b = ¾
From the K or the Cl: a = b + c => 1 = ¾ + c => c = ¼
Result
1KClO3 → ¾ KClO4 + ¼KCl
Or multiplying by 4: 4KClO3 → 3KClO4 + KCl
| Atom | Number in reactants | Number in products |
| K | 4 | 4 |
| Cl | 4 | 4 |
| O | 12 | 12 |
————————————————————
14. Al2(SO4)3 + Ca(OH)2 → Al(OH)3 + CaSO4
Answer: Al2(SO4)3 + 3Ca(OH)2 → 2Al(OH)3 + 3CaSO4
Steps:
Note: SO42- and OH– ionic components
1Al2(SO4)3 + Ca(OH)2 → Al(OH)3 + CaSO4
1Al2(SO4)3 + Ca(OH)2 → 2Al(OH)3 + CaSO4
1Al2(SO4)3 + Ca(OH)2 → 2Al(OH)3 + 3CaSO4
1Al2(SO4)3 + 3Ca(OH)2 → 2Al(OH)3 + 3CaSO4
1Al2(SO4)3 + 3Ca(OH)2 → 2Al(OH)3 + 3CaSO4 (not needed, checking OH–)
Al2(SO4)3 + 3Ca(OH)2 → 2Al(OH)3 + 3CaSO4
| Atom | Number in reactants | Number in products |
| Al | 2 | 2 |
| SO42- | 3 | 3 |
| Ca | 3 | 3 |
| OH– | 6 | 6 |
————————————————————
15. H2SO4 + HI → H2S + I2 + H2O
Answer: H2SO4 + 8HI → H2S + 4I2 + 4H2O
Steps:
1H2SO4 + HI → H2S + I2 + H2O
1H2SO4 + HI → 1H2S + I2 + H2O
1H2SO4 + HI → 1H2S + I2 + 4H2O
1H2SO4 + 8HI → 1H2S + I2 + 4H2O
1H2SO4 + 8HI → 1H2S + 4I2 + 4H2O
H2SO4 + 8HI → H2S + 4I2 + 4H2O
As this equation is a bit challenging, using the algebra approach might make it easier:
aH2SO4 + bHI → cH2S + dI2 + eH2O
Equations:
H: 2a + b = 2c + 2e
S: a = c
O: 4a = e
I: b = 2d
Resolution:
Fix a = 1
From S: a = c => c = 1
From O: 4a = e => 4×1 = e => e = 4
From H: 2a + b = 2c + 2e => 2×1 + b = 2×1 + 2×4 => 2 + b = 10 => b = 8
From I: b = 2d => 8 = 2d => d = 4
Answer:
1H2SO4 + 8HI → 1H2S + 4I2 + 4H2O
H2SO4 + 8HI → H2S + 4I2 + 4H2O
| Atom | Number in reactants | Number in products |
| H | 10 | 10 |
| S | 1 | 1 |
| O | 4 | 4 |
| H | 10 | 10 |
| I | 8 | 8 |
————————————————————
16. Al + O2 → Al2O3
Answer: 4Al + 3O2 → 2Al2O3
Steps:
2Al + O2 → Al2O3 (As I have 2 Al on the Al2O3, it seems a good choice to use 2)
2Al + O2 → 1Al2O3
2Al + 3/2O2 → 1Al2O3
4Al + 3O2 → 2Al2O3
4Al + 3O2 → 2Al2O3
| Atom | Number in reactants | Number in products |
| Al | 4 | 4 |
| O | 6 | 6 |
————————————————————
17. Al(NO3)3 + NaOH → Al(OH)3 + NaNO3
Answer: Al(NO3)3 + 3NaOH → Al(OH)3 + 3NaNO3
Steps:
1Al(NO3)3 + NaOH → Al(OH)3 + NaNO3
1Al(NO3)3 + NaOH → 1Al(OH)3 + NaNO3
1Al(NO3)3 + NaOH → 1Al(OH)3 + 3NaNO3
1Al(NO3)3 + 3NaOH → 1Al(OH)3 + 3NaNO3
1Al(NO3)3 + 3NaOH → 1Al(OH)3 + 3NaNO3 (Not needed, checking OH–)
Al(NO3)3 + 3NaOH → Al(OH)3 + 3NaNO3
| Atom | Number in reactants | Number in products |
| Al | 1 | 1 |
| NO3– | 3 | 3 |
| OH– | 3 | 3 |
| Na | 3 | 3 |
————————————————————
18. O2 + CS2 →CO2 + SO2
Answer: 3O2 + CS2 →CO2 + 2SO2
Steps:
O2 + 1CS2 →CO2 + SO2 (S and C appear 1 reactants and 1 product)
O2 + 1CS2 →1CO2 + SO2
O2 + 1CS2 →1CO2 + 2SO2
3O2 + 1CS2 →1CO2 + 2SO2
3O2 + CS2 →CO2 + 2SO2
| Atom | Number in reactants | Number in products |
| O | 6 | 6 |
| C | 1 | 1 |
| S | 2 | 2 |
————————————————————
19. BaF2 + K3PO4 → Ba3(PO4)2 + KF
Answer: 3BaF2 + 2K3PO4 → Ba3(PO4)2 + 6KF
Steps:
BaF2 + K3PO4 → 1Ba3(PO4)2 + KF
3BaF2 + K3PO4 → 1Ba3(PO4)2 + KF
3BaF2 + 2K3PO4 → 1Ba3(PO4)2 + KF
3BaF2 + 2K3PO4 → 1Ba3(PO4)2 + 6KF
3BaF2 + 2K3PO4 → 1Ba3(PO4)2 + 6KF (Steps not needed, checking F)
3BaF2 + 2K3PO4 → Ba3(PO4)2 + 6KF
| Atom | Number in reactants | Number in products |
| Ba | 3 | 3 |
| F | 6 | 6 |
| K | 6 | 6 |
| PO43- | 2 | 2 |
————————————————————
20. H2SO4 + Mg(NO3)2→ MgSO4 + HNO3
Answer: H2SO4 +Mg(NO3)2→ MgSO4 + 2HNO3
Steps:
H2SO4 +1Mg(NO3)2→ MgSO4 + HNO3
H2SO4 +1Mg(NO3)2→ 1MgSO4 + HNO3
H2SO4 +1Mg(NO3)2→ 1MgSO4 + 1HNO3
1H2SO4 +1Mg(NO3)2→ 1MgSO4 + 1HNO3
H2SO4 +Mg(NO3)2→ MgSO4 + 2HNO3
| Atom | Number in reactants | Number in products |
| H | 2 | 2 |
| SO42- | 1 | 1 |
| Mg | 1 | 1 |
| NO3– | 2 | 2 |
====================================================================
Comments
If you have any question, or want to add anything else, please leave a comment below. Also, if you have a chemical equation difficult you can post it below, we can try to solve it.
