On this page, I explain how to balance reduction-oxidation (redox) chemical equations. You should be familiarized with the basic balancing equations, which I explain in this link.
Outline for this page:
Redox equations
I have created a video explaining how to balance redox equations. You can watch it by clicking here.
These reactions are more complex than simple reactions. You will see then after you have seen the simple reactions. In these reactions, the oxidation states of the atoms change (more below). In some cases, the equations can be solved as shown in the simple equations, however, on other cases (like ionic equations in aqueous solutions) it is simpler to use half equations (as it is used in redox equations).
A Redox reaction is a reaction that involve the transfer of electrons between 2 species. For example:
2Na(s) + Cl2(g) → 2NaCl(s)
It is a redox equation, where the Na loses an electron:
Na → Na+ + 1e–
And the Cl gains an electron:
Cl2 + 2e– → 2Cl–
Definitions:
- Oxidation: is the reaction of losing electrons (eg: Na → Na+ + 1e–)
- Reduction: is the reaction of gaining electrons (eg: Cl2 + 2e– → 2Cl–)
- Oxidant, oxidizing agent or oxidizer is a component that oxidize other elements (in the example, the Cl2 is the oxidant of the Na)
- Reducer or reducing agent is a component that reduce other elements (in the example, the Na is the reducer of the Cl2)
To easy memorize the definition of Oxidation and Reduction is to use the mnemonic word OIL RIG:
OIL RIG: Oxidation Is Losing, Reduction Is Gaining.
The 2 most common methods to balance redox equation are the oxidation number and the ion-electron method. I personally find the latter easier, as it is more systematic and can solve more complex equations.
Oxidation state
Oxidation state is the hypothetical charge of an atom if all of its bond to other atoms were fully ionic. The oxidation state is used to balance ionic equations. The following rules simplify the determination of the oxidation state:
- The oxidation state of an individual atom is zero (as it has not been oxidised or reduced yet). For example, Cl2, Fe, H2, S8 have all oxidation state zero
- The sum of the oxidation states of all the atoms or ions in a neutral compound is zero.
- The sum of the oxidation states of all the atoms in an ion is equal to the charge of the ion.
- The more electronegative element in a substance is given a negative oxidation state. The less electronegative one is given a positive oxidation state. (Fluorine is the most electronegative element followed by oxygen). A table with the electronegativities of the different elements can be found at Electronegativities of the elements (data page) – Wikipedia,
- Fluorine: always -1
- Oxygen its oxidation state is -2, except in the peroxides and oxygen fluorides that is -1
- Peroxides (like H2O2, Na2O2, BaO2, …), where the 2 atoms of Oxygen are linked together (R-O-O-R) and the oxidation estate is -1
- Oxygen fluorides (like OF2O, O2F2, …): as the Fluorine is more electronegative that the Oxygen, the Oxygen the oxidation state is -1
- Chlorine: usually -1. Except in compounds with oxygen and fluorine, in these cases it is better to work out, as its oxidation states can be +1 (case of Cl2O), +3 (case of NaClO2), +4 (case of ClO2), +5 (case of NaClO3) and +7 (Cl2O7)
- Group 1, alkali metals, (when part of a molecule) has the oxidation state +1 (there are very rare exceptions to this rule and form what is known as ‘Alkalide’, discovered in 1974 by Prof James L Dye[1], for most common purposes, the rule of + 1 applies)
- Group 2, alkali earth metals, the oxidation estate is +2
[1] J. L. Dye; J. M. Ceraso; Mei Lok Tak; B. L. Barnett; F. J. Tehan (1974). “Crystalline salt of the sodium anion (Na−)”. Journal of the American Chemical Society 96 (2): 608–609
Examples to find oxidation states:
1. Find the oxidation state of the sulphur in sulfuric acid, H2SO4.
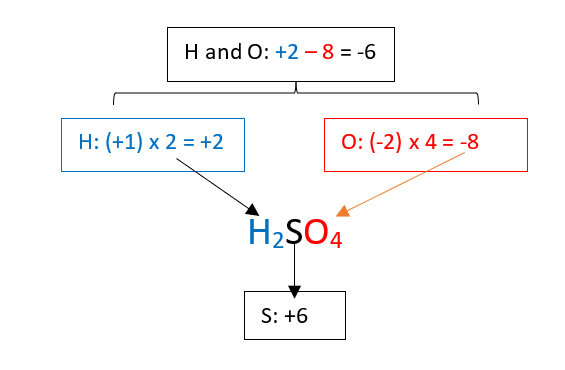
- As we have 2 hydrogen atoms, each with an oxidation state (+1), therefore, their total contribution is (+1) x 2 = +2
- As we have 4 oxygen atoms, each with an oxidation state (-2), therefore their total contribution is (-2) x 4 = -8
- The total contribution of hydrogen and oxygen atoms is + 2 – 8 = -6
- As the molecule is neutral, then, the sulphur needs to compensate for -6, therefore it oxidation state is +6
I find sometimes easier to use a mathematical equation to find the oxidation states (calling x to the unknown):
Total oxidation state of H + Total oxidation state of S + Total oxidation state of O = Total oxidation state H2SO4
2(+1) + x + 4(-2) = 0
2 + x – 8 = 0
x = 8 – 2
x = +6
Answer: +6
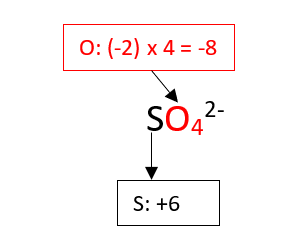
2. Find the oxidation state of the sulfur in the sulfate ion, SO42-.
As seen in the previous example, the total contribution of the oxygen is -8 and the total charge of the ion is -2 (there are no hydrogen atoms on this ion). Therefore, the S compensate only for 6 negative charges, this means that its oxidation state is +6.
Or using again a mathematical equation:
Total oxidation state of S + Total oxidation state of O = Total oxidation state SO42-
x + 4(-2) = -2
x – 8 = 0
x = 8 – 2
x = +6
Answer: +6
3. Find oxidation state of chromium in calcium dichromate, CaCr2O7
- The calcium has an oxidation state of +2, as it is only 1 atom of calcium, its total contribution is therefore: (+2) x 1 = +2
- There are 7 atoms of oxygen, each with an oxidation state of -2, therefore their total contribution is (-2) x 7 = -14
- The total contribution of the calcium and oxygen atoms is +2 – 14 = -12
- As the molecule is neutral, the 2 atoms of chromium need to compensate the charge of -12, therefore, each chromium has an oxidation state of +6
Or using again a mathematical equation:
Total oxidation state of Ca + Total oxidation state of Cr + Total oxidation state O = Total oxidation state CaCr2O7
1(+2) + 2x + 7(-2) = 0
2 + 2x – 14 = 0
2x = 14 – 2
2x = +12
x = +12/2
x = +6
Answer: +6
4. Find the oxidation state of chlorine in magnesium chloride, MgCl2.
- The atom of magnesium has an oxidation state of +2
- As the magnesium chloride is neutral, the 2 atoms of chlorine need to compensate the +2 from the magnesium, therefore each atom of chloride has an oxidation state of -1
Or using a mathematical equation:
Total oxidation state of Mg + Total oxidation state of Cl = Total oxidation state MgCl2
1(+2) + 2x = 0
2 + 2x = 0
2x = – 2
x = -2/2
x = -1
Answer: -1
5. Find the oxidation state of chlorine in the chlorous acid, HClO2
This is one example where the chlorine has an oxidation state different of -1, as the oxygen is more electronegative than the chlorine, the oxygen takes the electrons from the chlorine.
- Hydrogen: 1 atom with an oxidation state +1
- Oxygen: 2 atoms, each with an oxidation (-2) therefore their contribution is 2 x (-2) = -4
- The oxygen and hydrogen atoms have a total contribution of 1 – 4 = -3
- As the chlorous acid is neutral, the chlorine has an oxidation state of +3 (to compensate the -3 from the oxygen and hydrogen atoms)
Or using a mathematical equation:
Total oxidation state of H + Total oxidation state of Cl + Total oxidation state O = Total oxidation state HClO2
1(+1) + x + 2(-2)= 0
1 + x -4 = 0
x = +4 -1
x = +3
Answer: +3
Steps to adjust redox reactions
The steps to follow will depend on if the reaction is taking place in a neutral solution, in acidic aqueous solutions or in basic (alkaline) aqueous solutions. The straighter forward method is in case of neutral solutions, the other two cases require additional steps.
In neutral solutions
These are the easiest to adjust, and you can treat them as simple reactions, i.e. don’t need to use half-equations. The steps are:
- Identify the elements that are reduced and oxidised. Write their half-reaction (i.e.: the reduction reaction and oxidation reaction) balancing the elements in the reaction and the relevant number of electrons (in the oxidation reaction the electrons will appear as products and in the reduction reaction the electron will appear as reactants)
- Adjust both half-equations to ensure that the number of electrons are the same.
- Add both half-equations
- The same number of electrons should appear as reactants than as products, cancelling each other.
Example
Balance the following:
Cu+(aq) + Fe(s) → Fe3+(aq) + Cu(s)
In this example the cupric ion (Cu+) is reduced to copper (Cu) and the iron (Fe) is oxidised to ferric ion (Fe3+). The half-equations are:
Cu+(aq) + 1e–→ Cu(s)
Fe(s) →Fe3+(aq) + 3e–
To balance the electrons in both equations, we multiply by 3 the copper reaction:
3Cu+(aq) + 3e–→ 3Cu(s)
Now we add both half-equations:
3Cu+(aq) + 3e–→ 3Cu(s)
Fe(s) →Fe3+(aq) + 3e–
———————————————————
3Cu+(aq) + 3e–+ Fe(s)→ 3Cu(s) + Fe3+(aq) + 3e–
Cancelling the electrons in both sides:
3Cu+(aq) + Fe(s)→ 3Cu(s) + Fe3+(aq)
Let’s check that the equation is balanced:
| Atom | Number in reactants | Number in products |
| Cu | 3 | 3 |
| Fe | 1 | 1 |
| Charges | 3+ | 3+ |
In acidic aqueous solutions
In these equations we are going to add, in this order, water (H2O), hydrogen ions (also referred as protons, H+) and electrons (e–)
- Identify the elements that are reduced and oxidised.
- Write their half-reaction balancing the elements except for the hydrogen (H) and the oxygen (O).
- Balance the oxygen atoms by adding the appropriate number of water (H2O) molecules to the opposite side of the equation.
- Balance the hydrogen atoms (including those added in the previous step) by adding protons (H+) to the opposite side of the equation.
- Add up the charges on each side and add electrons (e–) to equalize the charges in both sides of each half-equation.
- Compare the number of electrons in each half-equation, if the number is different, multiply by a convenient number (it helps to find the lowest common multiple) to make them equal.
- Add both half-equations
- Cancel the electrons (that should be the same number on both sides)
- Cancel any common terms in reactants and in products
Example
Balance the reaction in acidic solution of MnO4–(aq) + I– (aq) → Mn2+(aq) + I2(s)
Identification of the reduction and oxidation half-reactions
Reduction: MnO4–(aq) → Mn2+(aq)
(The manganese changes from oxidation state +7 to +2)
Oxidation: I–(aq) → I2(s)
(The iodine changes from oxidation state -1 to 0)
Balancing the number of atoms, except for the H and O:
Reduction: MnO4–(aq) → Mn2+(aq)
(There is one manganese on each side of the equation)
Oxidation: 2I–(aq) → I2(s)
(I need 2 iodine ions to form 1 molecule of iodine)
Balancing the O, where it is necessary, by adding H2O:
Reduction: MnO4–(aq) → Mn2+(aq) + 4H2O(l)
(I need 4 atoms of oxygen in the products)
Oxidation: 2I–(aq) → I2(s)
(No oxygen in this half-equation)
Balancing the H, where it is necessary, by adding H+:
Reduction: MnO4–(aq) + 8H+(aq) → Mn2+(aq) + 4H2O(l)
(I need 8 atoms of hydrogen in the reactants)
Oxidation: 2I–(aq) → I2(s)
(No oxygen in this half-equation)
Balancing the charges in each half-equation by adding e–:
Reduction: MnO4–(aq) + 8H+(aq) + 5e–→ Mn2+(aq) + 4H2O(l)
(Reactants have a total charge of 7+, the products 2+, to balance both sides, we need to add 5 e– to the reactants and have both sides a total charge of 2+)
Oxidation: 2I–(aq) → I2(s) + 2e–
(Reactants have a total charge of 0, the products 2-, to balance both sides, we need to add 2 e– to the products and have both sides a total charge of 2-)
Making the electrons of each half-equation equal–:
Reduction: (multiplying by 2): 2MnO4–(aq) + 16H+(aq) + 10e–→ 2Mn2+(aq) + 8H2O(l)
Oxidation: (multiplying by 5): 10I–(aq) → 5I2(s) + 10e–
(Multiplying by 2 the reduction half-equation (2 is the number of electrons in the oxidation half-equation) and by 5 the oxidation half-equation (5 is the number of electrons in the reduction half-equation) I obtain 10 electrons on both half-equations)
Adding both half-equations equal–:
Reduction: 2MnO4–(aq) + 16H+(aq) + 10e–→ 2Mn2+(aq) + 8H2O(l)
Oxidation: 10I–(aq) → 5I2(s) + 10e–
———————————————————————————
Result: 2MnO4–(aq) + 16H+(aq) + 10e– + 10I(aq)– → 2Mn2+(aq) + 8H2O(l) + 5I2(s) + 10e–
Cancelling electrons (10 on each side)
2MnO4–(aq) + 16H+(aq) + 10e– + 10I(aq)– → 2Mn2+(aq) + 8H2O(l) + 5I2(s) + 10e-
2MnO4–(aq) + 16H+(aq) + 10I–(aq) → 2Mn2+(aq) + 8H2O(l) + 5I2(s)
There are no other common terms to cancel
Let’s check the number of atoms and charges:
| Atom | Number in reactants | Number in products |
| Mn | 2 | 2 |
| O | 8 | 8 |
| H | 16 | 16 |
| I | 10 | 10 |
| Charges | 4+ | 4+ |
In basic (alkaline) aqueous solutions
The steps are the same than in the acidic solution until step 9 (cancelling common terms on both sides of the equation). The steps to follow are:
- Identify the elements that are reduced and oxidised.
- Write their half-reaction balancing the elements except for the hydrogen (H) and the oxygen (O).
- Balance the oxygen atoms by adding the appropriate number of water (H2O) molecules to the opposite side of the equation.
- Balance the hydrogen atoms (including those added in the previous step) by adding protons (H+) to the opposite side of the equation.
- Add up the charges on each side and add electrons (e–) to equalize the charges in both sides of each half-equation.
- Compare the number of electrons in each half-equation, if the number is different, multiply by a convenient number to made them equal.
- Add both half-equations
- Cancel the electrons (that should be the same number in both sides)
- Add, in both sides of the equation, as many OH– (hydroxide ions) as H+ there are in the final equation.
- Each OH– and each H+ on the same side of the equation cancel each other to form 1 molecule of H2O. This should leave the final equation with OH– and H2O and no H+ (if this is not the case, review what was done in the previous step)
- Cancel any other common terms in reactants and in products
Note: an alternative method to this is to balance the oxygen (step 3) by adding H2O and OH–, excess of H+ are balanced by adding more OH–, however, I found this alternative method more complex on some occasions, the method described above is more straight forward.
Example
Balance the reaction in basic solution of Ag(s) + Zn2+(aq) → Ag2O(aq) + Zn(s)
Identification of the reduction and oxidation half-reactions
Reduction: Zn2+(aq) → Zn(s)
(The zinc changes from oxidation state +2 to 0)
Oxidation: Ag(s) → Ag2O(aq)
(The silver changes from oxidation 0 to +1)
Balancing the number of atoms, except for the H and O:
Reduction: Zn2+(aq) → Zn(s)
(The zinc is balanced)
Oxidation: 2Ag(s) → Ag2O(aq)
(We need to atoms of silver as reactant)
Balancing the O, where it is necessary, by adding H2O:
Reduction: Zn2+(aq) → Zn(s)
(No oxygen is required with the zinc)
Oxidation: 2Ag(s) + H2O(l)→ Ag2O(aq)
(As in the products we have 1 oxygen, we add 1 molecule of water as reactant)
Balancing the H, where it is necessary, by adding H+:
Reduction: Zn2+(aq) → Zn(s)
(No hydrogen is required with the zinc)
Oxidation: 2Ag(s) + H2O(l) → Ag2O(aq) + 2H+(aq)
(As in the reactants we have 2 hydrogens and none in the products, we add 2 H+ in the products)
Balancing the charges in each half-equation by adding e–:
Reduction: Zn2+(aq) + 2e– → Zn(s)
(We have 2 positive charges in the reactants and no charges in the products. We balance it by adding 2 electrons in the reactants)
Oxidation: 2Ag(s) + H2O(l) → Ag2O(aq) + 2H+(aq) + 2e–
(As in the reactants we have no charges in the products 2 positive charges, we balance the equation by adding 2 electrons in the products)
Making the electrons of each half-equation equal–:
Reduction: Zn2+(aq) + 2e– → Zn(s)
Oxidation: 2Ag(s) + H2O → Ag2O(aq) + 2H+(aq) + 2e–
(In both equations we have the same number of electrons, not need to modify)
Adding both half-equations equal–:
Reduction: Zn2+(aq) + 2e– → Zn(s)
Oxidation: 2Ag(s) + H2O → Ag2O(aq) + 2H+(aq) + 2e–
———————————————————————————————–
Result: Zn2+(aq) + 2e– + 2Ag(s) + H2O → Zn(s) + Ag2O(aq) + 2H+(aq) + 2e–
Cancelling electrons (2 on each side)
Zn2+(aq) + 2e– + 2Ag(s) + H2O → Zn(s) + Ag2O(aq) + 2H+ + 2e-
Zn2+(aq) + 2Ag(s) + H2O → Zn(s) + Ag2O(aq) + 2H+(aq)
Adding OH‑ to compensate for the H+
Zn2+(aq) + 2Ag(s) + H2O + 2OH‑(aq) → Zn(s) + Ag2O(aq) + 2H+(aq) + 2OH‑(aq)
(As we have 2 H+ as products, we add 2 OH– in the products and in the reactants)
Forming 1 molecule of H2O with each OH– and each H+ on the same side of the equation
Zn2+(aq) + 2Ag(s) + H2O + 2OH‑(aq) → Zn(s) + Ag2O(aq) + 2H2O(l)
(As we have 2 H+ and 2 OH– in the products, we form 2 H20)
Cancelling common terms
Zn2+(aq) + 2Ag(s) + H2O(l) + 2OH‑(aq) → Zn(s) + Ag2O(aq) + 2H2O(l) + H2O(l)
(We have 1 molecule of water as reactant and 2 molecules of water as product, we cancel 1 molecule of water in the products)
Result:
Zn2+(aq) + 2Ag(s) + 2OH‑(aq) → Zn(s) + Ag2O(aq) + H2O(l)
Checking the atoms and charges:
| Atom | Number in reactants | Number in products |
| Zn | 1 | 1 |
| Ag | 2 | 2 |
| O | 4 | 4 |
| H | 2 | 2 |
| Charges | 0 | 0 |
Exercises
List of exercises. Click in the number of the exercise to go to the exercise.
| Number | Question | Answer | Type |
|---|---|---|---|
| 1 | S2O32-(aq) + I3–(aq) → S4O62-(aq) + I–(aq) | 2S2O32-(aq) + I3–(aq) → S4O62-(aq) + 3I–(aq) | Neutral |
| 2 | Cr2O72-(aq) + NO2–(aq) → Cr3+(aq) + NO3−(aq) | Cr2O72-(aq) + 3NO2–(aq) + 8H+(aq) →2Cr3+(aq) + 3NO3−(aq) + 4H2O(l) | Acid |
| 3 | Fe2+(aq) + MnO4–(aq) → 5Fe3+(aq) + Mn2+(aq) | 5Fe2+(aq) + MnO4–(aq) + 8H+(aq) → 5Fe3+(aq) + Mn2+(aq) + 4H2O(l) | Acid |
| 4 | MnO4−(aq) + I−(aq) → I2 (s)+ Mn2+(aq) | 2MnO4−(aq) + 16H+(aq) + 10I−(aq)→ 2Mn2+(aq) + 5I2(s) + 8H2O(l) | Acid |
| 5 | MnO4−(aq) + I−(aq) → I2 (s)+ Mn2+(aq) | 2MnO4−(aq) + 8H2O(l) + 10I−(aq)→ 2Mn2+(aq) + 5I2(s) + 16OH– (aq) | Base |
| 6 | Cr2O72−(aq)+ C2H5OH(l) → Cr3+(aq) + CO2(g) | 2Cr2O72− (aq)+ 16H+(aq) + C2H5OH(l) → 4Cr3+(aq) + 11H2O(l) + 2CO2(g) | Acid. |
| 7 | Cr2O72−(aq) + C2H5OH(l) → Cr3+(aq) + CO2(g) | 2Cr2O72−(aq)+ 5H2O(l) + C2H5OH(l) → 4Cr3+ + 16OH–(aq)+ 2CO2(g) | Base |
| 8 | Cl2(g) → Cl–(aq) + ClO3–(aq) | 3H2O(l) + 3Cl2(g) → 5Cl–(aq) + ClO3–(aq) + 6H+(aq) | Acid |
| 9 | Cl2(g) → Cl–(aq) + ClO3–(aq) | 6OH–(aq) + 3Cl2(g) → 5Cl–(aq) + ClO3–(aq) + 3H2O(l) | Basic |
| 10 | MnO4–(aq) + H2C2O4(aq) → Mn2+(aq) + CO2(g) | 2MnO4–(aq) + 5H2C2O4(aq) + 6H+(aq) → 2Mn2+(aq) + 8H2O(aq) + 10CO2(g) | Acid |
| 11 | Cr2O72-(aq) + Fe2+(aq) → Cr3+(aq) + Fe3+(aq) | Cr2O72-(aq) + 6Fe2+(aq) + 14H+(aq) → 2Cr3+(aq) + 6Fe3+(aq) + 7H2O(l) | Acid |
| 12 | MnO4–(aq) + H2SO3(aq) → Mn2+(aq) + SO42-(aq) | 2MnO4–(aq) + 5H2SO3(aq) → 2Mn2+(aq) + 5SO42-(aq) + 3H2O(l) + 4H+(aq) | Acid |
| 13 | MnO4–(aq) + SO32-(aq) → MnO2(s) + SO42-(aq) | 2MnO4–(aq) + 3SO32-(aq) + H2O(l) → 2MnO2(s) + 3SO42-(aq) + 2OH–(aq) | Base |
| 14 | MnO4–(aq) + Br–(aq) → MnO2(s) + BrO3–(aq) | 2MnO4–(aq) + Br–(aq) + H2O(l) → 2MnO2(s) + BrO3–(aq) + 2OH–(aq) | Base |
| 15 | Cu(s) + NO3–(aq) → Cu2+(aq) + NO(g) | 3Cu(aq) + 2NO3–(aq) + 8H+(aq) → 3Cu2+(aq) + 2NO(g) + 4H2O(l) | Acid |
| 16 | SO42-(aq) + Zn(s) → H2S(g) + Zn2+(aq) | SO42-(aq) + 4Zn(s) + 10H+(aq) → H2S(g) + 4Zn2-(aq) + 4H2O(l) | Acid |
| 17 | SO42-(aq) + Cu(s) → SO2(g) + Cu2+(aq) | SO42–(aq) + Cu(s) + 4H+(aq) → SO2(g) + Cu2+(aq) + 2H2O(l) | Acid |
1. Balance the equation S2O32-(aq) + I3–(aq) → S4O62-(aq) + I–(aq) in neutral conditions
Answer: 2S2O32-(aq) + I3–(aq) → S4O62-(aq) + 3I–
Half equations:
Oxidation: 2S2O32-(aq) → S4O62-(aq) + 2e–
Reduction: I3–(aq) + 2e–→3I–(aq)
As the electron are already equalized, we can add both equations
2S2O32-(aq) → S4O62-(aq) + 2e–
I3–(aq) + 2e–→3I–(aq)
————————————————-
2S2O32-(aq) + I3–(aq) + 2e–→ S4O62-(aq) + 2e–(aq)+ 3I–
And cancel the electrons:
2S2O32-(aq) + I3–(aq) → S4O62-(aq) + 3I–
| Atom | Number in reactants | Number in products |
| S | 4 | 4 |
| O | 6 | 6 |
| I | 3 | 3 |
| Charges | 5 | 5 |
2. Balance the equation in acidic conditions: Cr2O72-(aq) + NO2–(aq) →Cr3+(aq) + NO3-(aq)
Answer: Cr2O72-(aq) + 3NO2–(aq) + 8H+(aq) →2Cr3+(aq) + 3NO3−(aq) + 4H2O(l)
Half equations:
Reduction: Cr2O72-(aq) →Cr3+(aq)
Oxidation: NO2–(aq) → NO3−(aq)
Adjusting elements except O and H:
Reduction: Cr2O72-(aq) →2Cr3+(aq)
Oxidation: NO2–(aq) → NO−3(aq)
Adjusting O by adding H2O:
Reduction: Cr2O72-(aq) →2Cr3+(aq) + 7 H2O(l)
Oxidation: H2O(l) + NO2–(aq) → NO3−(aq)
Adjusting H by adding H+:
Reduction: 14H+(aq) + Cr2O72-(aq) → 2Cr3+(aq) + 7H2O(l)
Oxidation: H2O(l) + NO2–(aq) → NO3−(aq) + 2H+(aq)
Equalizing charges by adding e–:
Reduction: 14H+(aq) + Cr2O72-(aq) + 6e– →2Cr3+(aq) + 7H2O(l)
Oxidation: H2O(l) + NO2–(aq) → NO3−(aq) + 2H+(aq) + 2e–
Equalizing electrons in both half-equations:
Reduction: 14H+(aq) + Cr2O72-(aq) + 6e– →2Cr3+(aq) + 7H2O(l)
Oxidation: 3H2O(l) + 3NO2–(aq) → 3NO3−(aq) + 6H+(aq) + 6e–
Adding both half-equations:
Reduction: 14H+(aq) + Cr2O72-(aq) + 6e– →2Cr3+(aq) + 7H2O(l)
Oxidation: 3H2O(l) + 3NO2–(aq) → 3NO3−(aq) + 6H+(aq) + 6e–
———————————————————————————
Full equation: 14H+(aq) + Cr2O72-(aq) + 6e– + 3H2O(l) + 3NO2–(aq) →2Cr3+(aq) + 7H2O(l) + 3NO3−(aq) + 6H+(aq) + 6e–
Cancelling electrons
14H+(aq) + Cr2O72-(aq) + 6e– + 3H2O(l) + 3NO2–(aq) →2Cr3+(aq) + 7H2O(l) + 3NO3−(aq) + 6H+(aq) + 6e–
14H+(aq) + Cr2O72-(aq) + 3H2O(l) + 3NO2–(aq) →2Cr3+(aq) + 7H2O(l) + 3NO3−(aq) + 6H+(aq)
Cancelling common terms:
14H+(aq) + Cr2O72-(aq) + 3H2O(l) + 3NO2–(aq) →2Cr3+(aq) + 7H2O(l) + 3NO3−(aq) + 6H+(aq)
8H+(aq) + Cr2O72-(aq) + 3NO2–(aq) →2Cr3+(aq) + 4H2O(l) + 3NO3−(aq)
We obtain:
8H+(aq) + Cr2O72-(aq) + 3NO2–(aq) →2Cr3+(aq) + 4H2O(l) + 3NO3−(aq)
The equation is balanced, but I prefer to leave H+ and H2O at the end, therefore I rearrange the equation
Cr2O72-(aq) + 3NO2–(aq) + 8H+(aq) →2Cr3+(aq) + 3NO3−(aq) + 4H2O(l)
| Atom | Number in reactants | Number in products |
| Cr | 2 | 2 |
| O | 7 + 3×2 =13 | 4 + 3×3 = 9 |
| N | 3 | 3 |
| H | 8 | 4×2 = 8 |
| Charges | 8-2-3 =3+ | 3×2 – 3 = 3+ |
3. Balance the equation in acidic conditions: MnO4–(aq) + Fe2+(aq) → Mn2+(aq) + Fe3+(aq)
Answer: 5Fe2+(aq) + MnO4–(aq) + 8H+(aq) → 5Fe3+(aq) + Mn2+(aq) + 4H2O(l)
Half equations:
Oxidation: Fe2+(aq) → Fe3+(aq)
Reduction: MnO4–(aq) → Mn2+(aq)
Adjusting O by adding H2O:
Oxidation: Fe2+(aq) → Fe3+(aq)
Reduction: MnO4–(aq) → Mn2+(aq) + 4H2O(l)
Adjusting H by adding H+:
Oxidation: Fe2+(aq) → Fe3+(aq)
Reduction: MnO4–(aq) + 8H+(aq)→ Mn2+(aq) + 4H2O(l)
Adjusting charges by adding e–:
Oxidation: Fe2+(aq) → Fe3+(aq) + 1e–
Reduction: MnO4–(aq) + 8H+(aq) + 5e–→ Mn2+(aq) + 4H2O(l)
Equalizing electrons:
Oxidation: 5 x (Fe2+(aq) → Fe3+(aq) + 1e–)
5Fe2+(aq) → 5Fe3+(aq) + 5e–
Reduction: 1 x (MnO4–(aq) + 8H+(aq) + 5e–→ Mn2+(aq) + 4H2O(l))
MnO4–(aq) + 8H+(aq) + 5e–→ Mn2+(aq) + 4H2O(l)
Adding half-equations:
Oxidation: 5Fe2+(aq) → 5Fe3+(aq) + 5e–
Reduction: MnO4–(aq) + 8H+(aq) + 5e–→ Mn2+(aq) + 4H2O(l)
——————————————————————————-
Equation: 5Fe2+(aq) + MnO4–(aq) + 8H+(aq) + 5e–→ 5Fe3+(aq) + 5e– + Mn2+(aq) + 4H2O(l)
Eliminating electrons and common terms:
5Fe2+(aq) + MnO4–(aq) + 8H+(aq) + 5e– → 5Fe3+(aq) + 5e– + Mn2+(aq) + 4H2O(l)
Result:
5Fe2+(aq) + MnO4–(aq) + 8H+(aq) → 5Fe3+(aq) + Mn2+(aq) + 4H2O(l)
| Atom | Number in reactants | Number in products |
| Fe | 5 | 5 |
| Mn | 1 | 1 |
| O | 4 | 4 |
| H | 8 | 4×2 = 8 |
| Charges | 5×2 – 1 + 8 = 17+ | 5×3 + 2 = 17+ |
4. Balance in acidic conditions the reaction: MnO4−(aq) + I−(aq) → I2 (s)+ Mn2+(aq)
Answer: 2MnO4−(aq) + 16H+(aq) + 10I−(aq)→ 2Mn2+(aq) + 5I2(s) + 8H2O(l)
Half reactions:
Reduction: MnO4− → Mn2+
Oxidation: I− → I2
Adjusting elements except O and H:
Reduction: MnO4− → Mn2+
Oxidation: 2I− → I2
Adjusting O by adding H2O:
Reduction: MnO4− → Mn2+ + 4H2O
Oxidation: 2I− → I2
Adjusting H by adding H+:
Reduction: MnO4− + 8H+ → Mn2+ + 4H2O
Oxidation: 2I− → I2
Equalizing charges by adding e–:
Reduction: MnO4− + 8H+ + 5e–→ Mn2+ + 4H2O
Oxidation: 2I− → I2 + 2e–
Equalizing electrons in both half-equations:
Reduction: 2 x (MnO4− + 8H+ + 5e–→ Mn2+ + 4H2O)
2MnO4− + 16H+ + 10e–→ 2Mn2+ + 8H2O
Oxidation: 5 x (2I− → I2 + 2e–)
10I− → 5I2 + 10e–
Adding both half-equations:
Reduction: 2MnO4− + 16H+ + 10e–→ 2Mn2+ + 8H2O
Oxidation: 10I− → 5I2 + 10e–
————————————————————-
Full equation: 2MnO4− + 16H+ + 10e– + 10I−→ 2Mn2+ + 8H2O + 5I2 + 10e–
Cancelling electrons common terms(there are no other common terms to cancel):
2MnO4− + 16H+ + 10e– + 10I−→ 2Mn2+ + 8H2O + 5I2 + 10e–
Result
2MnO4− + 16H+ + 10I−→ 2Mn2+ + 8H2O + 5I2
| Atom | Number in reactants | Number in products |
| Mn | 2 | 2 |
| O | 8 | 8 |
| H | 16 | 16 |
| I | 10 | 10 |
| Charges | 4+ | 4+ |
5. Balance in basic (alkaline) conditions the reaction: MnO4− + I− → I2 + Mn2+
Answer: 2MnO4−(aq) + 8H2O(l) + 10I−(aq)→ 2Mn2+(aq) + 5I2(s) + 16OH– (aq)
The equation to balance is the same than in exercise 4, but we have changed the conditions from acid to basic. As you can see, the steps are the same, but we modify the final equation by adding, in both sides of the equation, as many OH– as H+ there are in the final equation.
Half reactions:
Reduction: MnO4− → Mn2+
Oxidation: I− → I2
Adjusting elements except O and H:
Reduction: MnO4− → Mn2+
Oxidation: 2I− → I2
Adjusting O by adding H2O:
Reduction: MnO4− → Mn2+ + 4H2O
Oxidation: 2I− → I2
Adjusting H by adding H+:
Reduction: MnO4− + 8H+ → Mn2+ + 4H2O
Oxidation: 2I− → I2
Equalizing charges by adding e–:
Reduction: MnO4− + 8H+ + 5e–→ Mn2+ + 4H2O
Oxidation: 2I− → I2 + 2e–
Equalizing electrons in both half-equations:
Reduction: 2 x (MnO4− + 8H+ + 5e–→ Mn2+ + 4H2O)
2MnO4− + 16H+ + 10e–→ 2Mn2+ + 8H2O
Oxidation: 5 x (2I− → I2 + 2e–)
10I− → 5I2 + 10e–
Adding both half-equations:
Reduction: 2MnO4− + 16H+ + 10e–→ 2Mn2+ + 8H2O
Oxidation: 10I− → 5I2 + 10e–
————————————————————-
Full equation: 2MnO4− + 16H+ + 10e– + 10I−→ 2Mn2+ + 8H2O + 5I2 + 10e–
Cancelling electrons common terms(there are no other common terms to cancel):
2MnO4− + 16H+ + 10e– + 10I−→ 2Mn2+ + 8H2O + 5I2 + 10e–
Result
2MnO4− + 16H+ + 10I−→ 2Mn2+ + 8H2O + 5I2
The additional steps, as it is alkaline conditions, is to add, in both sides of the equation, as many OH– as H+ there are in the final equation. As we have 16H+, we add 16OH–:
2MnO4− + 16H+ + 16OH– + 10I−→ 2Mn2+ + 8H2O + 16OH– + 5I2
On the left we have “16H+ + 16OH– ” that will react giving water: 16H+ + 16OH– → 16H2O
2MnO4− + 16H2O + 10I−→ 2Mn2+ + 8H2O + 16OH– + 5I2
Eliminating the excess of water:
2MnO4− + 16H2O + 10I−→ 2Mn2+ + 8H2O + 16OH– + 5I2
2MnO4− + 8H2O + 10I−→ 2Mn2+ + 16OH– + 5I2
Therefore the answer is:
2MnO4− + 8H2O + 10I−→ 2Mn2+ + 16OH– + 5I2
Checking that the balance is correct:
| Atom | Number in reactants | Number in products |
| Mn | 2 | 2 |
| O | 16 | 16 |
| H | 16 | 16 |
| I | 10 | 10 |
| Charges | 12- | 12- |
6. Balance in acidic conditions Cr2O72−(aq) + C2H5OH(l) → Cr3+(aq) + CO2(g)
Answer: 2Cr2O72− + 16H+ + C2H5OH → 4Cr3+ + 11H2O + 2CO2
Half reactions:
Reduction: Cr2O72− → Cr3+
Oxidation: C2H5OH → CO2
Adjusting elements except O and H:
Reduction: Cr2O72− → 2Cr3+
Oxidation: C2H5OH → 2CO2
Adjusting O by adding H2O:
Reduction: Cr2O72− → 2Cr3+ + 7H2O
Oxidation: C2H5OH + 3H2O → 2CO2
Adjusting H by adding H+:
Reduction: Cr2O72− + 14H+→ 2Cr3+ + 7H2O
Oxidation: C2H5OH + 3H2O→ 2CO2 + 12H+
Equalizing charges by adding e–:
Reduction: Cr2O72− + 14H+ + 6e–→ 2Cr3+ + 7H2O
Oxidation: C2H5OH + 3H2O → 2CO2 + 12H++ 12e–
Equalizing electrons in both half-equations:
Reduction: 2x(Cr2O72− + 14H+ + 6e–→ 2Cr3+ + 7H2O)
2Cr2O72− + 28H+ + 12e–→ 4Cr3+ + 14H2O
Oxidation: C2H5OH + 3H2O → 2CO2 + 12H++ 12e–
Adding both half-equations:
Reduction: 2Cr2O72− + 28H+ + 12e–→ 4Cr3+ + 14H2O
Oxidation: C2H5OH + 3H2O → 2CO2 + 12H++ 12e–
————————————————————————
Full equation: 2Cr2O72− + 28H+ + 12e– + C2H5OH + 3H2O → 4Cr3+ + 14H2O 2CO2 + 12H++ 12e
Cancelling electrons common terms:
2Cr2O72− + 28H+ + 12e– + C2H5OH + 3H2O → 4Cr3+ + 11H2O + 2CO2 + 12H++ 12e
2Cr2O72− + 16H+ + C2H5OH → 4Cr3+ + 11H2O + 2CO2
Result:
2Cr2O72− + 16H+ + C2H5OH → 4Cr3+ + 11H2O + 2CO2
| Atom | Number in reactants | Number in products |
| Cr | 4 | 4 |
| O | 15 | 15 |
| H | 22 | 22 |
| C | 2 | 2 |
| Charges | 12+ | 12+ |
7. Balance in basic conditions Cr2O72−(aq) + C2H5OH(l) → Cr3+(aq) + CO2(g)
Answer: 2Cr2O72−(aq)+ 5H2O(l) + C2H5OH(l) → 4Cr3+ + 16OH–(aq)+ 2CO2(g)
The steps are the same than in exercise 6, with additional steps after we obtain the final answer. Refer to exercise 6 to see the development until the final result:
2Cr2O72− + 16H+ + C2H5OH + 3H2O → 4Cr3+ + 14H2O + 2CO2
The additional steps, as it is alkaline conditions, is to add, in both sides of the equation, as many OH– as H+ there are in the final equation. As we have 16H+, we add 16OH–:
2Cr2O72− + 16H+ + 16OH–+ C2H5OH + 3H2O → 4Cr3+ + 14H2O + + 16OH–+ 2CO2
As 16H+ + 16OH– → H20
2Cr2O72− + 16H2O + C2H5OH + 3H2O → 4Cr3+ + 14H2O + 16OH–+ 2CO2
We eliminate the excess of water:
2Cr2O72− + 16H2O + C2H5OH + 3H2O → 4Cr3+ + 14H2O + 16OH–+ 2CO2
2Cr2O72− + 5H2O + C2H5OH → 4Cr3+ + 14H2O + 16OH–+ 2CO2
Result: 2Cr2O72− + 5H2O + C2H5OH → 4Cr3+ + 16OH–+ 2CO2
| Atom | Number in reactants | Number in products |
| Cr | 4 | 4 |
| O | 20 | 20 |
| H | 16 | 16 |
| C | 2 | 2 |
| Charges | 4- | 4- |
8. Balance in acidic conditions Cl2(g) → Cl–(aq) + ClO3–(aq)
This is a case of disproportionation or dismutation, in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states
Answer: 3H2O(l) + 3Cl2(g) → 5Cl–(aq) + ClO3–(aq) + 6H+(aq)
Half reactions:
Reduction: Cl2 → Cl–
Oxidation: Cl2 → ClO3–
Adjusting elements except O and H:
Reduction: Cl2 → 2Cl–
Oxidation: Cl2 → 2ClO3–
Adjusting O by adding H2O:
Reduction: Cl2 → 2Cl–
Oxidation: 6H2O + Cl2 → 2ClO3–
Adjusting H by adding H+:
Reduction: Cl2 → 2Cl–
Oxidation: 6H2O + Cl2 → 2ClO3– + 12H+
Equalizing charges by adding e–:
Reduction: 2e– + Cl2 → 2Cl–
Oxidation: 6H2O + Cl2 → 2ClO3– + 12H+ + 10e–
Equalizing electrons in both half-equations:
Reduction: 5 x (2e– + Cl2 → 2Cl–)
10e– + 5Cl2 → 10Cl–
Oxidation: 6H2O + Cl2 → 2ClO3– + 12H+ + 10e–
Adding both half-equations:
Reduction: 10e– + 5Cl2 → 10Cl–
Oxidation: 6H2O + Cl2 → 2ClO3– + 12H+ + 10e –
———————————————————
Full equation: 10e– + 5Cl2 + 6H2O + Cl2 → 10Cl– 2ClO3– + 12H+ + 10e –
Cancelling electrons and common terms:
10e– + 5Cl2 + 6H2O + Cl2 → 10Cl– + 2ClO3– + 12H+ + 10e –
6H2O + 6Cl2 → 10Cl– + 2ClO3– + 12H+
As all the coefficients are even, we can divide by 2 all of them and have a more simplified version:
3H2O + 3Cl2 → 5Cl– + ClO3– + 6H+
Result: 3H2O + 3Cl2 → 5Cl– + ClO3– + 6H+
| Atom | Number in reactants | Number in products |
| Cl | 6 | 6 |
| H | 6 | 6 |
| O | 3 | 3 |
| Charges | 0 | 0 |
9. Balance in basic conditions Cl2(g) → Cl–(aq) + ClO3–(aq)
Answer: 6OH–(aq)+ 3Cl2(g) → 5Cl–(aq) + ClO3–(aq) + 3H2O(l)
This is a case of disproportionation or dismutation, in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. The steps are the same than the previous exercise, where we have the answer:
3H2O + 3Cl2 → 5Cl– + ClO3– + 6H+
Adding OH– in each side of the equation as H+ we have:
6OH– + 3H2O + 3Cl2 → 5Cl– + ClO3– + 6H+ + 6OH–
Forming H2O from H+ and OH–:
6OH– + 3H2O + 3Cl2 → 5Cl– + ClO3– + 6H+ + 6OH–
6OH– + 3H2O + 3Cl2 → 5Cl– + ClO3– + 6H2O
Removing excess of water
6OH– + 3H2O + 3Cl2 → 5Cl– + ClO3– + 6H2O
6OH– + 3Cl2 → 5Cl– + ClO3– + 3H2O
Answer: 6OH– + 3Cl2 → 5Cl– + ClO3– + 3H2O
| Atom | Number in reactants | Number in products |
| Cl | 6 | 6 |
| H | 6 | 6 |
| O | 6 | 6 |
| Charges | 6 | 6 |
10. Balance in acidic conditions MnO4–(aq) + H2C2O4(aq) → Mn2+(aq) + CO2(g)
Answer: 2MnO4–(aq) + 5H2C2O4(aq) + 6H+(aq)→ 2Mn2+(aq)+ 8H2O(l) + 10CO2(g)
Half reactions:
Reduction: MnO4– → Mn2+
Oxidation: H2C2O4 → CO2
Adjusting elements except for O and H:
Reduction: MnO4– → Mn2+
Oxidation: H2C2O4 → 2CO2
Adjusting O by adding H2O:
Reduction: MnO4– → Mn2+ + 4H2O
Oxidation: H2C2O4 → 2CO2
Adjusting H by adding H+:
Reduction: 8H+ + MnO4– → Mn2+ + 4H2O
Oxidation: H2C2O4 → 2CO2 + 2H+
Equalizing charges by adding e–:
Reduction: 5e– + 8H+ + MnO4– → Mn2+ + 4H2O
Oxidation: H2C2O4 → 2CO2 + 2H+ + 2e–
Equalizing electrons in both half-equations:
Reduction: 2 x (5e– + 8H+ + MnO4– → Mn2+ + 4H2O)
10e– + 16H+ + 2MnO4– → 2Mn2+ + 8H2O
Oxidation: 5 x (H2C2O4 → 2CO2 + 2 H+ + 2e–)
5H2C2O4 → 10CO2 + 10H+ + 10e–
Adding both half-equations:
Reduction: 10e– + 16H+ + 2MnO4– → 2Mn2+ + 8H2O
Oxidation: 5H2C2O4 → 10CO2 + 10H+ + 10e–
————————————————————————-
Full equation: 10e– + 16H+ + 2MnO4– + 5H2C2O4 → 2Mn2+ + 8H2O + 10CO2– + 10H+ + 10e–
Cancelling electrons common terms:
10e– + 16H+ + 2MnO4– + 5H2C2O4 → 2Mn2+ + 8H2O + 10CO2 + 10H+ + 10e–
6H+ + 2MnO4– + 5H2C2O4 → 2Mn2+ + 8H2O + 10CO2–
Result
2MnO4–(aq) + 5H2C2O4(aq) + 6H+(aq) → 2Mn2+(aq) + 8H2O(aq) + 10CO2(g)
| Atom | Number in reactants | Number in products |
| Mn | 2 | 2 |
| O | 28 | 28 |
| C | 10 | 10 |
| H | 16 | 16 |
| Charges | 4+ | 4+ |
11. Balance in acidic conditions Cr2O72-(aq) + Fe2+(aq) → Cr3+(aq) + Fe3+(aq)
Answer: Cr2O72-(aq) + 6Fe2+(aq) + 14H+(aq) → 2Cr3+(aq) + 6Fe3+(aq) + 7H2O(l)
Half reactions:
Reduction: Cr2O72- → Cr3+
Oxidation: Fe2+ → Fe3+
Adjusting elements except for O and H:
Reduction: Cr2O72- → 2Cr3+
Oxidation: Fe2+ → Fe3+
Adjusting O by adding H2O:
Reduction: Cr2O72- → 2Cr3+ + 7H2O
Oxidation: Fe2+ → Fe3+
Adjusting H by adding H+:
Reduction: 14H+ + Cr2O72- → 2Cr3+ + 7H2O
Oxidation: Fe2+ → Fe3+
Equalizing charges by adding e–:
Reduction: 6e– + 14H+ + Cr2O72- → 2Cr3+ + 7H2O
Oxidation: Fe2+ → Fe3+ + e–
Equalizing electrons in both half-equations:
Reduction: 6e– + 14H+ + Cr2O72- → 2Cr3+ + 7H2O
Oxidation: 6 x (Fe2+ → Fe3+ + e–)
6Fe2+ → 6Fe3+ + 6e–
Adding both half-equations:
Reduction: 6e– + 14H+ + Cr2O72- → 2Cr3+ + 7H2O
Oxidation: 6Fe2+ → 6Fe3+ + 6e–
—————————————————————–
Full equation: : 6e– + 14H+ + Cr2O72- + 6Fe2+ → 2Cr3+ + 7H2O + 6Fe3+ + 6e–
Cancelling electrons common terms:
6e– + 14H+ + Cr2O72- + 6Fe2+ → 2Cr3+ + 7H2O + 6Fe3+ + 6e–
14H+ + Cr2O72- + 6Fe2+ → 2Cr3+ + 7H2O + 6Fe3+
Result
Cr2O72-(aq) + 6Fe2+(aq) + 14H+(aq) → 2Cr3+(aq) + 6Fe3+(aq) + 7H2O(l)
| Atom | Number in reactants | Number in products |
| Cr | 2 | 2 |
| O | 7 | 7 |
| Fe | 6 | 6 |
| H | 14 | 14 |
| Charges | 24+ | 24+ |
12. Balance in acidic conditions MnO4–(aq) + H2SO3(aq) → Mn2+(aq) + SO42-(aq)
Answer: 2MnO4–(aq) + 5H2SO3(aq) → 2Mn2+(aq) + 5SO42-(aq) + 3H2O(l) + 4H+(aq)
Half reactions:
Reduction: MnO4– → Mn2+
Oxidation: H2SO3 → SO42-
Adjusting elements except for O and H (no change):
Reduction: MnO4– → Mn2+
Oxidation: H2SO3 → SO42-
Adjusting O by adding H2O:
Reduction: MnO4– → Mn2+ + 4H2O
Oxidation: H2O + H2SO3 → SO42-
Adjusting H by adding H+:
Reduction: 8H+ + MnO4– → Mn2+ + 4H2O
Oxidation: H2O + H2SO3 → SO42- + 4H+
Equalizing charges by adding e–:
Reduction: 5e– + 8H+ + MnO4– → Mn2+ + 4H2O
Oxidation: H2O + H2SO3 → SO42- + 4H+ + 2e–
Equalizing electrons in both half-equations:
Reduction: 2 x (5e– + 8H+ + MnO4– → Mn2+ + 4H2O)
10e– + 16H+ + 2MnO4– → 2Mn2+ + 8H2O
Oxidation: 5 x (H2O + H2SO3 → SO42- + 4H+ + 2e–)
5H2O + 5H2SO3 → 5SO42- + 20H+ + 10e–
Adding both half-equations:
Reduction: 10e– + 16H+ + 2MnO4– → 2Mn2+ + 8H2O
Oxidation: 5H2O + 5H2SO3 → 5SO42- + 20H+ + 10e–
———————————————————————————-
Full equation: 10e– + 16H+ + 2MnO4– + 5H2O + 5H2SO3 → 2Mn2+ + 8H2O + 5SO42- + 20H+ + 10e–
Cancelling electrons common terms:
10e– + 16H+ + 2MnO4– + 5H2O + 5H2SO3 → 2Mn2+ + 8H2O + 5SO42- + 20H+ + 10e-
2MnO4– + 5H2SO3 → 2Mn2+ + 3H2O + 5SO42- + 4H+
Result
2MnO4–(aq) + 5H2SO3(aq) → 2Mn2+(aq) + 5SO42-(aq) + 3H2O(l) + 4H+(aq)
| Atom | Number in reactants | Number in products |
| Mn | 2 | 2 |
| O | 23 | 23 |
| H | 10 | 10 |
| S | 5 | 5 |
| Charges | 2- | 2- |
13. Balance in basic conditions MnO4–(aq) + SO32-(aq) → MnO2(s) + SO42-(aq)
Answer: 2MnO4–(aq) + 3SO32-(aq) + H2O(l) → 2MnO2(s) + 3SO42-(aq) + 2OH–(aq)
Half reactions:
Reduction: MnO4– → MnO2
Oxidation: SO32- → SO42-
Adjusting elements except for O and H (no change):
Reduction: MnO4– → MnO2
Oxidation: SO32- → SO42-
Adjusting O by adding H2O:
Reduction: MnO4– → MnO2 + 2H2O
Oxidation: H2O + SO32- → SO42-
Adjusting H by adding H+:
Reduction: 4H+ + MnO4– → MnO2 + 2H2O
Oxidation: H2O + SO32- → SO42- + 2H+
Equalizing charges by adding e–:
Reduction: 3e– + 4H+ + MnO4– → MnO2 + 2H2O
Oxidation: H2O + SO32- → SO42- + 2H+ + 2e–
Equalizing electrons in both half-equations:
Reduction: 2 x (3e– + 4H+ + MnO4– → MnO2 + 2H2O)
6e– + 8H+ + 2MnO4– → 2MnO2 + 4H2O
Oxidation: 3 x (H2O + SO32- → SO42- + 2H+ + 2e–)
3H2O + 3SO32- → 3SO42- + 6H+ + 6e–
Adding both half-equations:
Reduction: 6e– + 8H+ + 2MnO4– → 2MnO2 + 4H2O
Oxidation: 3H2O + 3SO32- → 3SO42- + 6H+ + 6e–
———————————————————————————-
Full equation: 6e– + 8H+ + 2MnO4– + 3H2O + 3SO32- → 2MnO2 + 4H2O + 3SO42- + 6H+ + 6e–
Cancelling electrons common terms:
6e– + 8H+ + 2MnO4– + 3H2O + 3SO32- → 2MnO2 + 4H2O + 3SO42- + 6H+ + 6e-
2H+ + 2MnO4– + 3SO32- → 2MnO2 + H2O + 3SO42-
Adding OH– in each side of the equation as H+ we have:
2OH– + 2H+ + 2MnO4– + 3SO32- → 2MnO2 + H2O + 3SO42- + 2OH–
Forming H2O from H+ and OH–:
2H2O + 2MnO4– + 3SO32- → 2MnO2 + H2O + 3SO42- + 2OH–
Removing excess of water
2H2O + 2MnO4– + 3SO32- → 2MnO2 + H2O + 3SO42- + 2OH–
H2O + 2MnO4– + 3SO32- → 2MnO2 + 3SO42- + 2OH–
Result:
2MnO4–(aq) + 3SO32-(aq) + H2O(l) → 2MnO2(s) + 3SO42-(aq) + 2OH–(aq)
| Atom | Number in reactants | Number in products |
| Mn | 2 | 2 |
| O | 18 | 18 |
| H | 2 | 2 |
| S | 3 | 3 |
| Charges | 8- | 8- |
14. Balance in basic conditions MnO4–(aq) + Br–(aq) → MnO2(s) + BrO3–(aq)
Answer: 2MnO4–(aq) + Br–(aq) + H2O(l) → 2MnO2(s) + BrO3–(aq) + 2OH–(aq)
Half reactions:
Reduction: MnO4– → MnO2
Oxidation: Br-‑→ BrO3–
Adjusting elements except for O and H (no change):
Reduction: MnO4– → MnO2
Oxidation: Br–→ BrO3–
Adjusting O by adding H2O:
Reduction: MnO4– → MnO2 + 2H2O
Oxidation: 3H2O + Br–→ BrO3–
Adjusting H by adding H+:
Reduction: 4H+ + MnO4– → MnO2 + 2H2O
Oxidation: 3H2O + Br‑→ BrO3–+ 6H+
Equalizing charges by adding e–:
Reduction: 3e– + 4H+ + MnO4– → MnO2 + 2H2O
Oxidation: 3H2O + Br‑→ BrO3–+ 6H+ + 6e–
Equalizing electrons in both half-equations:
Reduction: 2 x (3e– + 4H+ + MnO4– → MnO2 + 2H2O)
6e– + 8H+ + 2MnO4– → 2MnO2 + 4H2O
Oxidation: 3H2O + Br‑→ BrO3–+ 6H+ + 6e–
Adding both half-equations:
Reduction: 6e– + 8H+ + 2MnO4– → 2MnO2 + 4H2O
Oxidation: 3H2O + Br‑ → BrO3–+ 6H+ + 6e–
———————————————————————————-
Full equation: 6e– + 8H+ + 2MnO4– + 3H2O + Br‑ → 2MnO2 + 4H2O + BrO3– + 6H+ + 6e–
Cancelling electrons common terms:
6e– + 8H+ + 2MnO4– + 3H2O + Br‑ → 2MnO2 + 4H2O + BrO3– + 6H+ + 6e-
2H+ + 2MnO4– + Br– → 2MnO2 + H2O + BrO3–
Adding OH– in each side of the equation as H+ we have:
2OH– + 2H+ + 2MnO4– + Br– → 2MnO2 + H2O + BrO3– + 2OH–
Forming H2O from H+ and OH–:
2H2O + 2MnO4– + Br– → 2MnO2 + H2O + BrO3– + 2OH–
Removing excess of water
2H2O + 2MnO4– + Br– → 2MnO2 + H2O + BrO32- + 2OH–
H2O + 2MnO4– + Br– → 2MnO2 + BrO3– + 2OH–
Result:
2MnO4–(aq) + Br–(aq) + H2O(l) → 2MnO2(s) + BrO3–(aq) + 2OH–(aq)
| Atom | Number in reactants | Number in products |
| Mn | 2 | 2 |
| O | 9 | 9 |
| H | 2 | 2 |
| Br | 1 | 1 |
| Charges | 3- | 3- |
15. Balance in acidic conditions Cu(s) + NO3–(aq) → Cu2+(aq) + NO(g)
Answer: 3Cu(aq) + 2NO3–(aq) + 8H+(aq) → 3Cu2+(aq) + 2NO(g) + 4H2O(l)
Half reactions:
Reduction: NO3– → NO
Oxidation: Cu → Cu2+
Adjusting elements except for O and H (no change):
Reduction: NO3– → NO
Oxidation: Cu → Cu2+
Adjusting O by adding H2O:
Reduction: NO3– → NO + 2H2O
Oxidation: Cu → Cu2+
Adjusting H by adding H+:
Reduction: 4H+ + NO3– → NO + 2H2O
Oxidation: Cu → Cu2+
Equalizing charges by adding e–:
Reduction: 3e– + 4H+ + NO3– → NO + 2H2O
Oxidation: Cu → Cu2+ + 2e–
Equalizing electrons in both half-equations:
Reduction: 2 x (3e– + 4H+ + NO3– → NO + 2H2O)
6e– + 8H+ + 2NO3– → 2NO + 4H2O
Oxidation: 3 x (Cu → Cu2+ + 2e–)
3Cu → 3Cu2+ + 6e–
Adding both half-equations:
Reduction: 6e– + 8H+ + 2NO3– → 2NO + 4H2O
Oxidation: 3Cu → 3Cu2+ + 6e–
———————————————————————————-
Full equation: 6e– + 8H+ + 2NO3– + 3Cu → 2NO + 4H2O + 3Cu2+ + 6e–
Cancelling electrons common terms:
6e– + 8H+ + 2NO3– + 3Cu → 2NO + 4H2O + 3Cu2+ + 6e-
8H+ + 2NO3– + 3Cu → 2NO + 4H2O + 3Cu2+
Result
3Cu(aq) + 2NO3–(aq) + 8H+(aq) → 3Cu2+(aq) + 2NO(g) + 4H2O(l)
| Atom | Number in reactants | Number in products |
| Cu | 3 | 3 |
| N | 2 | 2 |
| O | 6 | 6 |
| H | 8 | 8 |
| Charges | 6+ | 6+ |
16. Balance in acidic conditions: SO42-(aq) + Zn(s) → H2S(g) + Zn2+(aq)
Answer: SO42–(aq) + 4Zn(s) + 10H+(aq) → H2S(g) + 4Zn2+(aq) + 4H2O(l)
Half reactions:
Reduction: SO42- → H2S
Oxidation: Zn → Zn2+
Adjusting elements except for O and H (no change):
Reduction: SO42- → H2S
Oxidation: Zn
Adjusting O by adding H2O:
Reduction: SO42- → H2S + 4H2O
Oxidation: Zn → Zn2+
Adjusting H by adding H+:
Reduction: SO42- + 10H+ → H2S + 4H2O
Oxidation: Zn → Zn2+
Equalizing charges by adding e–:
Reduction: SO42- + 10H+ + 8e–→ H2S + 4H2O
Oxidation: Zn → Zn2+ + 2e–
Equalizing electrons in both half-equations:
Reduction: SO42- + 10H+ + 8e–→ H2S + 4H2O
Oxidation: 4 x (Zn → Zn2+ + 2e–)
4Zn → 4Zn2+ + 8e–
Adding both half-equations:
Reduction: SO42- + 10H+ + 8e–→ H2S + 4H2O
Oxidation: 4Zn → 4Zn2+ + 8e–
———————————————————————————-
Full equation: SO42- + 10H+ + 8e– + 4Zn → H2S + 4H2O + 4Zn2+ + 8e–
Cancelling electrons and common terms and adding physical states:
SO42- + 10H+ + 8e– + 4Zn → H2S + 4H2O + 4Zn2+ + 8e–
Result (with indication of the physical states)
SO42–(aq) + 4Zn(s) + 10H+(aq) → H2S(g) + 4Zn2+(aq) + 4H2O(l)
| Atom | Number in reactants | Number in products |
| S | 1 | 1 |
| O | 4 | 4 |
| Zn | 4 | 4 |
| H | 10 | 10 |
| Charges | 8+ | 8+ |
17. Balance in acidic conditions: SO42-(aq) + Cu(s) → SO2(g) + Cu2+(aq)
Answer: SO42–(aq) + Cu(s) + 4H+(aq) → SO2(g) + Cu2+(aq) + 2H2O(l)
Half reactions:
Reduction: SO42- → SO2
Oxidation: Cu → Cu2+
Adjusting elements except for O and H (no change):
Reduction: SO42- → SO2
Oxidation: Cu → Cu2+
Adjusting O by adding H2O:
Reduction: SO42- → SO2 + 2H2O
Oxidation: Cu → Cu2+
Adjusting H by adding H+:
Reduction: SO42- + 4H+→ SO2 + 2H2O
Oxidation: Cu → Cu2+
Equalizing charges by adding e–:
Reduction: SO42- + 4H++ 2e–→ SO2 + 2H2O
Oxidation: Cu → Cu2++ 2e–
Equalizing electrons in both half-equations (no change):
Reduction: SO42- + 4H++ 2e–→ SO2 + 2H2O
Oxidation: Cu → Cu2++ 2e–
Adding both half-equations:
Reduction: SO42- + 4H++ 2e–→ SO2 + 2H2O
Oxidation: Cu → Cu2++ 2e–
———————————————————————————-
Full equation: SO42- + 4H+ + 2e– + Cu → SO2 +2H2O + + Cu2+ + 2e–
Cancelling electrons and common terms:
SO42- + 4H+ + 2e– + Cu → SO2 +2H2O + + Cu2+ + 2e–
Result (with indication of the physical states)
SO42–(aq) + Cu(s) + 4H+(aq) → SO2(g) + Cu2+(aq) + 2H2O(l)
| Atom | Number in reactants | Number in products |
| S | 1 | 1 |
| O | 4 | 4 |
| Cu | 1 | 1 |
| H | 4 | 4 |
| Charges | 2+ | 2+ |
Comments
If you have any question, or want to add anything else, please leave a comment below. Also, if you have a chemical equation difficult you can post it below, we can try to solve it.
