If you find this useful, please leave a comment at the end of the page.
A link to a video of this paper can be found in the link: OCR 2022 A Level Chemistry Paper 1 | Periodic Table, Elements & Physical Chemistry – YouTube

SECTION A
You should spend a maximum of 20 minutes on this section.
Write your answer to each question in the box provided.
Answer all the questions
1
An aqueous solution contains a mixture of chloride, bromide and iodide ions.
AgNO3(aq) is added to this mixture, followed by an excess of dilute NH3(aq).
The resulting mixture is then filtered.
Which compound(s) is/are present in the residue on the filter paper?
A AgCl only
B AgCl and AgBr
C AgBr only
D AgBr and AgI
Your answer
[1]

2
20 cm3 of nitrogen gas reacts with 10 cm3 of oxygen gas to form 20 cm3 of a gaseous product.
Which equation is the most likely for the reaction?
A N2(g) + O2(g) → 2NO(g)
B N2(g) + 2O2(g) → N2O4(g)
C 2N2(g) + O2(g) → 2N2O(g)
D 2N2(g) + 2O2(g) → 4NO(g)
Your answer
[1]

3
0.541 g of an element X is reacted with oxygen to form 0.790 g of the oxide X2O3.
What is the element X?
A Al
B Cr
C Ga
D Sc
Your answer
[1]


4
Hydrogen peroxide, H2O2, can be oxidised by manganate(VII) ions under acid conditions as shown below.
2MnO4–(aq) + 5H2O2(aq) + 6H+(aq) → 2Mn2+(aq) + 5O2(g) + 8H2O(l)
In a titration, 25.00 cm3 of a disinfectant containing hydrogen peroxide reacts with 22.00 cm3 of 0.125 mol dm–3 KMnO4(aq).
What is the concentration of H2O2, in mol dm–3, in the disinfectant?
Assume that KMnO4 only reacts with H2O2 in the disinfectant.
A 0.0440
B 0.110
C 0.275
D 0.550
Your answer
[1]

5
The mass of 4 molecules of a substance is 2.125 × 10–22 g.
What is the possible formula of the substance?
A CH4
B O2
C SO2
D I2
Your answer
[1]

6
Prussian blue, C18Fe7N18, is a deep blue pigment containing Fe2+, Fe3+ and CN− ions.
What are the numbers of Fe2+ and Fe3+ ions in one formula unit of C18Fe7N18?
A 2 Fe2+ and 5 Fe3+
B 3 Fe2+ and 4 Fe3+
C 4 Fe2+ and 3 Fe3+
D 5 Fe2+ and 2 Fe3+
Your answer
[1]

7
Bond enthalpies are shown in the table.
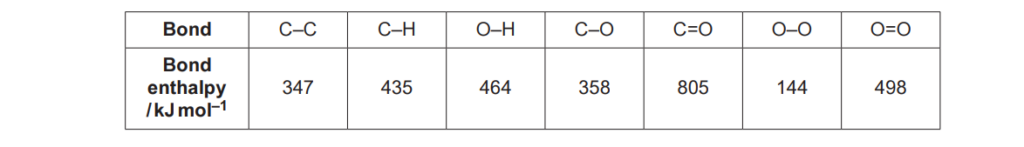
What is the enthalpy change, in kJ mol–1, for the reaction below?
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
A –730
B –544
C +544
D +730
Your answer
[1]
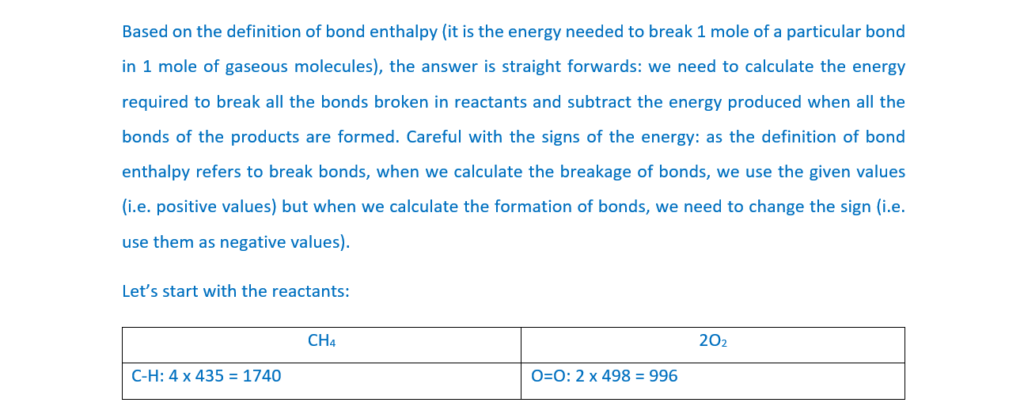
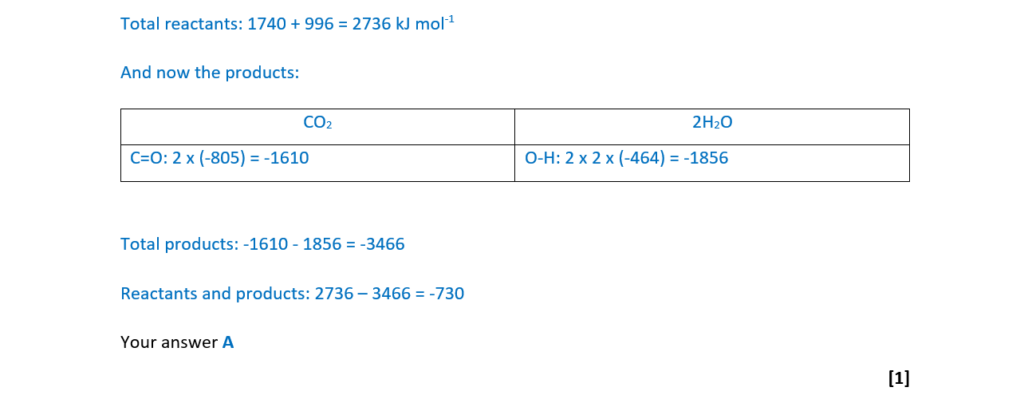
8
The half-life for a first order reaction is 80 s.
What is the rate constant k, in s–1, for this reaction?
A 8.66 × 10–3
B 0.0125
C 55.5
D 115
Your answer
[1]
9
Which equation represents the change that accompanies the standard enthalpy change of atomisation of bromine?
A ½ Br2(l) → Br(g)
B Br2(l) →2Br(g)
C ½ Br2(g) → Br(g)
D Br2(g) → 2Br(g)
Your answer
[1]

10
For the condensation of ammonia gas, what are the signs of ∆H and ∆S?
A ∆H –ve ∆S –ve
B ∆H –ve ∆S +ve
C ∆H +ve ∆S +ve
D ∆H +ve ∆S –ve

11
The equilibrium equation for an indicator, HA, is shown below.
Equation: HA(aq) ⇌ A–(aq) + H+(aq)
Colour: Blue Yellow
The indicator is added to a solution. The indicator turns a yellow colour.
An excess of aqueous sodium hydroxide is then added.
Which statement describes how the colour of this solution would be expected to change?
A Colour changes from yellow to blue.
B Colour changes from yellow to green.
C Colour changes from yellow to green and then to blue.
D Colour stays yellow.
Your answer
[1]

12
Ammonia and water react to set up an acid–base equilibrium.
What are the Brønsted–Lowry acids in the equilibrium mixture?
A H2O and OH–
B OH– and NH3
C NH4+ and H2O
D NH4+ and NH3
Your answer
[1]

13
This question was removed from the exam: H432-01 Post Exam Correction Jun22 (ocr.org.uk)
14
The three reactions below each form one product only.
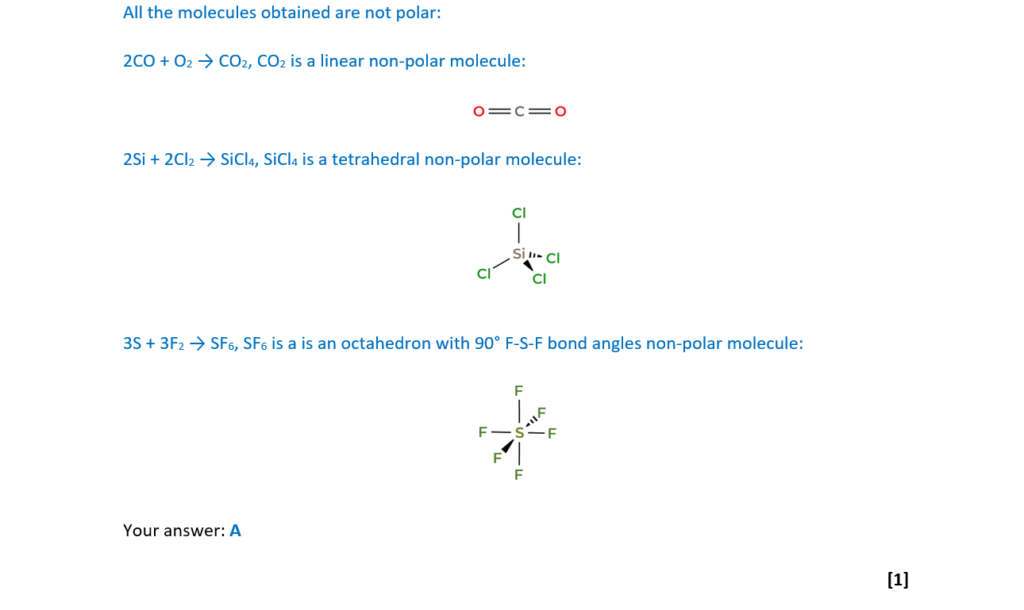
Which reaction(s) form(s) a product with non-polar molecules?
- 2CO + O2 →
- 2Si + 2Cl2 →
- 3S + 3F2 →
A 1, 2 and 3
B Only 1 and 2
C Only 2 and 3
D Only 1
Your answer
[1]
15
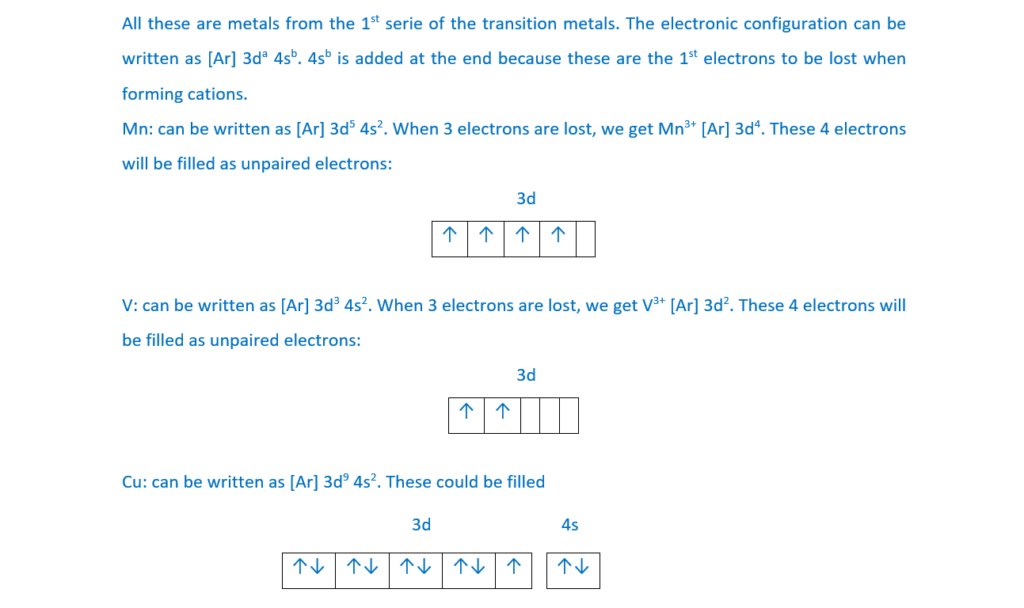
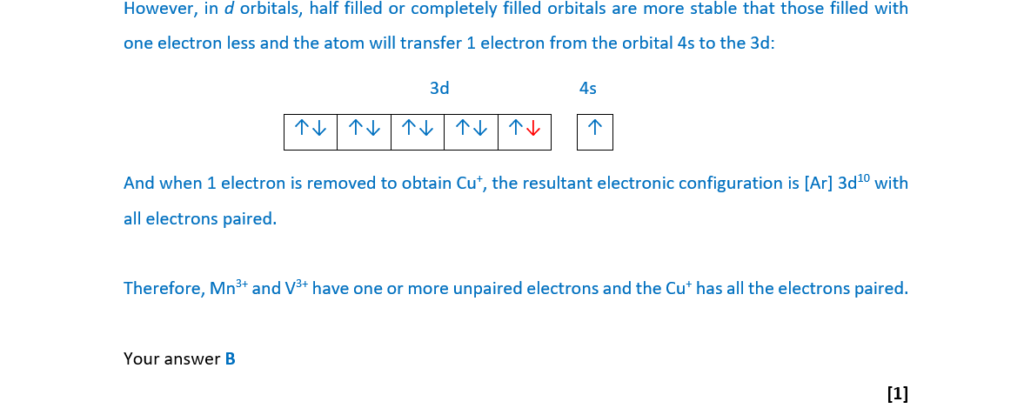
Which ion(s) contain(s) one or more unpaired electrons?
1 Mn3+
2 V3+
2 Cu+
A 1, 2 and 3
B Only 1 and 2
C Only 2 and 3
D Only 1
Your answer
[1]
SECTION B
Answer all the questions.
16
A catalytic converter in a car removes nitrogen monoxide, NO, and carbon monoxide, CO, from the exhaust gases.
(a)
One reaction that happens in a catalytic converter is shown below.
2CO(g) + 2NO(g) → N2(g) + 2CO2(g) Reaction 16.1
(i)
Explain how increasing the temperature increases the rate of Reaction 16.1.
Include a labelled sketch, using Boltzmann distributions, on the grid below.
Label the axes.
[3]
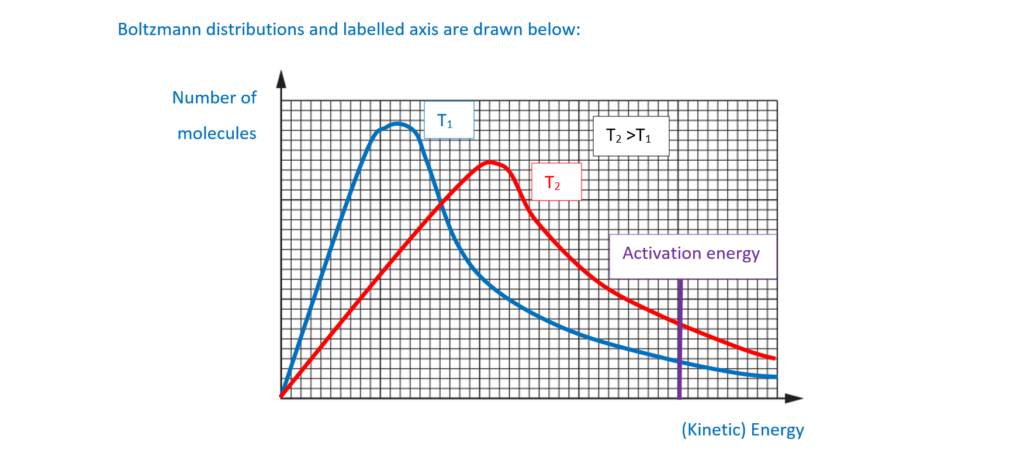

(ii)
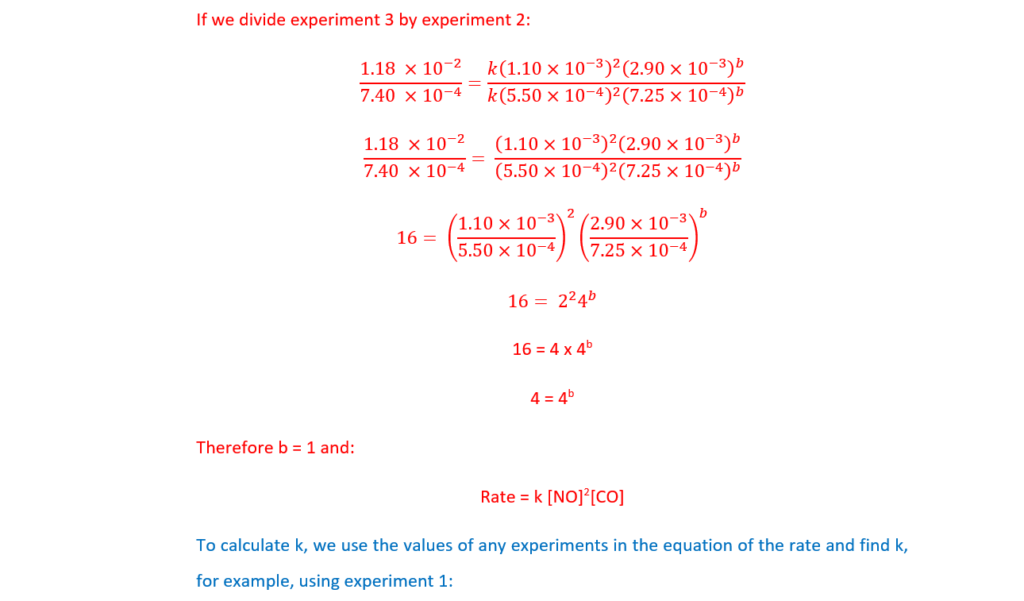
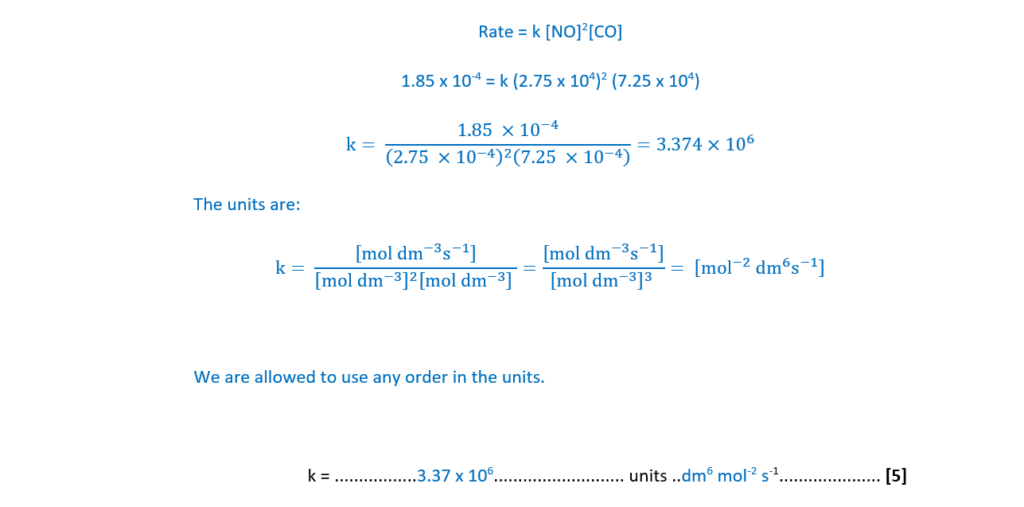
The rate of Reaction 16.1 is investigated by carrying out three experiments at the same temperature.
The results are shown below.
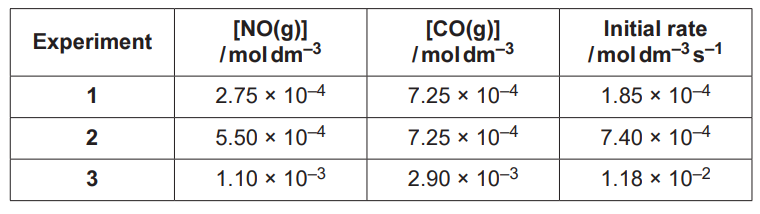
Determine the orders with respect to NO and CO, the rate equation, and the rate constant, k, including units.
Explain your reasoning.
k = …………………………………….. units ………………….. [5]

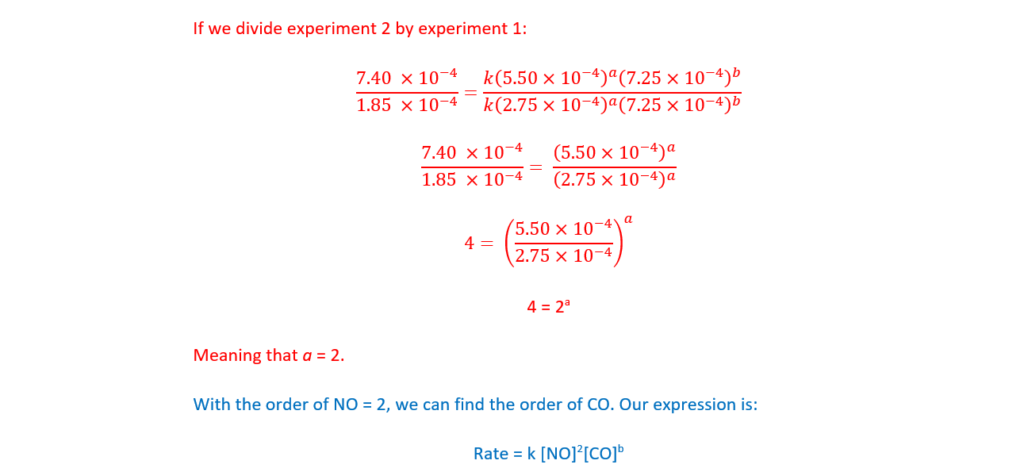
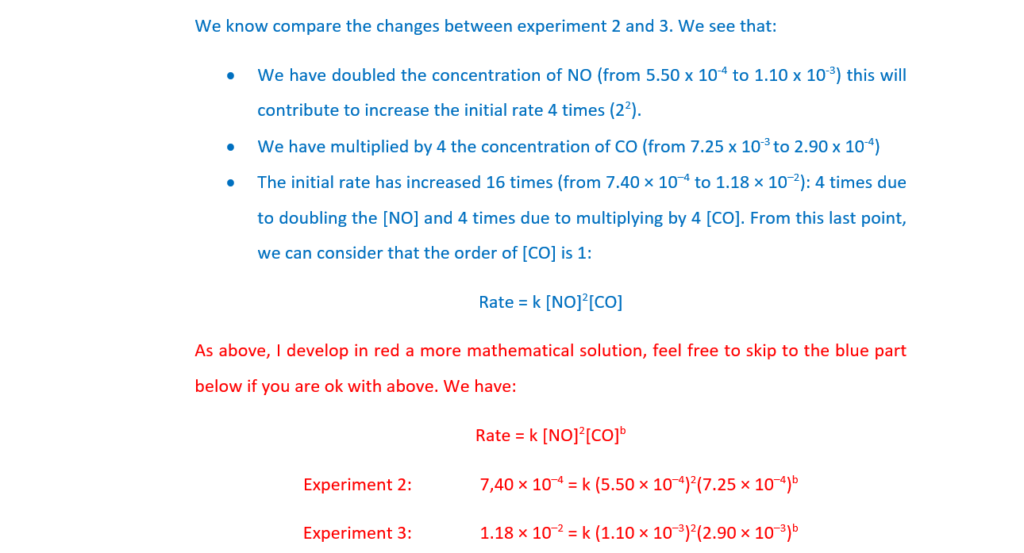
(b)
Carbon monoxide also reacts with nitrogen dioxide as shown in Reaction 16.2.
CO(g) + NO2(g) → NO(g) + CO2(g) Reaction 16.2
The rate equation for Reaction 16.2 is shown below:
rate = k[NO2(g)]2
Suggest a possible two-step mechanism for Reaction 16.2. The first step is much slower than the second step.
step 1 ………………………………………………………………………………………………………………………
step 2 ………………………………………………………………………………………………………………………
[2]


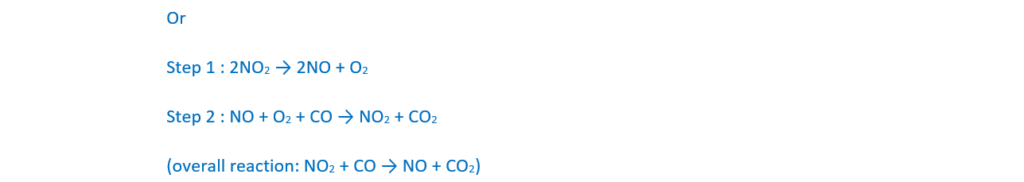
17
This question is about energy changes.
(a)
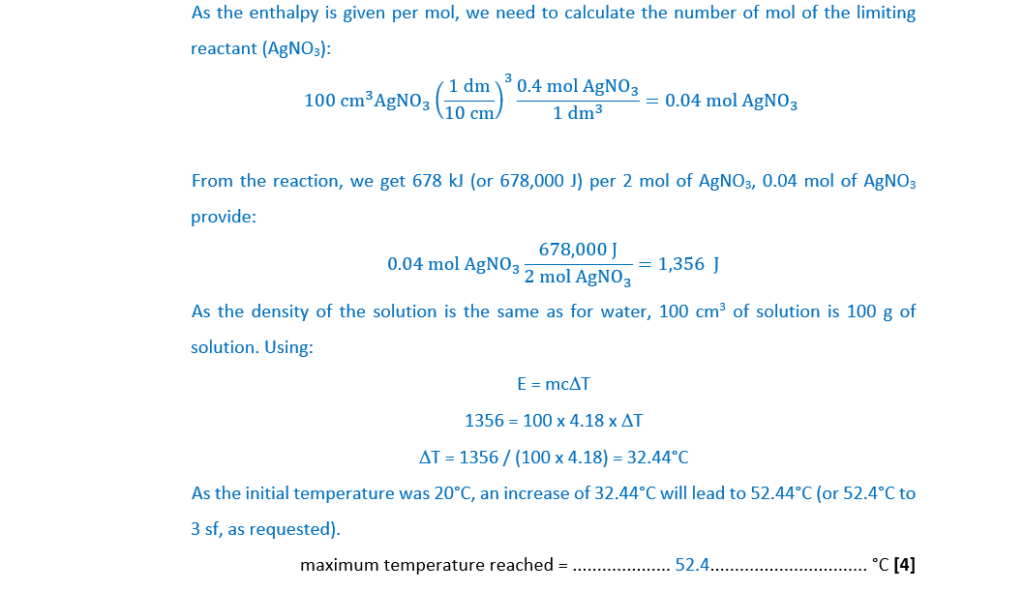
Magnesium reacts with aqueous silver nitrate, AgNO3(aq) as shown below.
Mg(s) + 2AgNO3(aq) → 2Ag(s) + Mg(NO3)2(aq) ∆H = –678 kJ mol–1
A student adds an excess of magnesium to 100.0 cm3 of 0.400 mol dm–3 AgNO3(aq).
The initial temperature is 20.0°C.
(i)
Determine the maximum temperature reached in this reaction.
Give your answer to 3 significant figures.
Assume that the specific heat capacity and density of the solution are the same as for water, and that there are no heat losses.
maximum temperature reached = ……………………………………………. °C [4]
(ii)
The student wants to repeat the experiment, but there is not enough AgNO3(aq) left to use another 100.0 cm3 portion.
The student decides to modify the method by adding an excess of magnesium to 50.0 cm3 of 0.400 mol dm–3 AgNO3(aq).
Predict, with reasons, how this modification would affect the maximum temperature reached.
Assume that there are no heat losses.
The temperature should be the same: half of the AgNO3(aq) have been used to increase the temperature of half of the solution,
[1]
(b)
Nitric acid is manufactured from ammonia in a multi-stage process.
The equation for the first stage in this process is shown in Reaction 17.1.
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(l) ∆Hө= –1172 kJ mol–1 Reaction 17.1
Some standard enthalpy changes of formation are shown in the table.
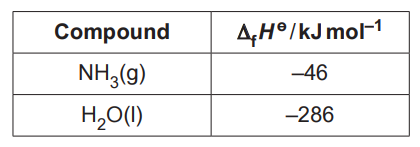
(i)
Explain the term enthalpy change of formation.
Enthalpy change of formation is the change of enthalpy when 1 mole of a compound is formed from its pure elements.
[1]
(ii)
Calculate the standard enthalpy change of formation, ∆fHө, of NO(g).
∆fHө of NO(g) = …………………………………….. kJ mol–1 [2]
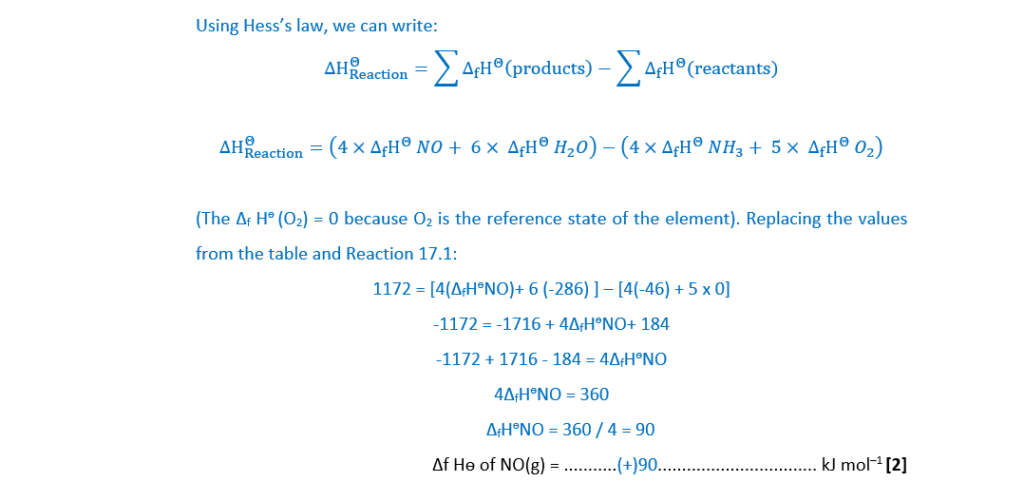
(c)
Carbon disulfide, CS2, reacts with dinitrogen oxide, N2O, as shown in Reaction 17.2.
4CS2(l) + 8N2O(g) → S8(s) + 4CO2(g) + 8N2(g) Reaction 17.2
Standard entropies, Sө, are shown in the table.

(i)
Explain the term entropy.
Entropy, S, is a measure of disorder or ‘the dispersal of energy’ within a system.
[1]
(ii)
The free energy change, ∆G, of Reaction 17.2 is –2672kJ mol–1 at 25°C.
Calculate the enthalpy change, ∆H, of Reaction 17.2, in kJ mol–1.
∆H = …………………………………….. kJ mol–1 [3]
(iii)
A student concludes that Reaction 17.2 is feasible at all temperatures. Explain whether the student is correct or not.
[2]

18
The graph shows the first ionisation energies for elements from helium, He, to boron, B, in the periodic table.
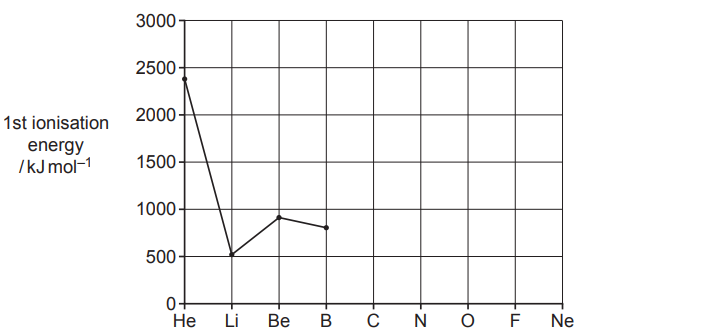
(a)
Complete the graph for C, N, O, F and Ne.
[2]

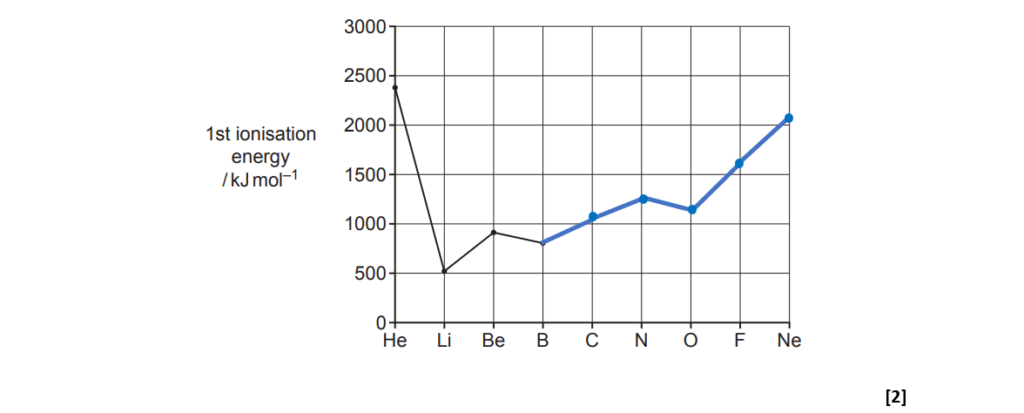
(b)
Estimate the energy required to form one Li+(g) ion from one Li(g) atom
.
Give your answer in kJ, in standard form, and to two significant figures
.
energy = …………………………………………….. kJ [1]

(c)
Explain why the first ionisation energies of He and Be are both higher than the first ionisation energy of Li.
Explanation for He: He is completing its first electronic shell (1s), the electron of Li is in the orbital 2s, subjected to shielding from the inner shell of electrons. He electrons experience greater nuclear attraction.
Explanation for Be: The shielding effect due to electrons form 1s is similar to Li, but there is an increase in the nuclear charge, giving greater attraction to the electron to be removed.
[4]
(d)
Explain why the first ionisation energy of Be is higher than the first ionisation energy of B.
The Be has an electronic configuration of 1s2, 2s2 and the B: 1s2, 2s2, 2p1 and electrons in 2p have higher energy than electrons in 2s, making it easier to remove.
[2]
19
This question is about acids and buffer solutions.
(a)
Succinic acid, HOOC(CH2)2COOH, is a weak dibasic acid that is used in tablet form in health supplements.
A student plans to determine the mass of succinic acid in one tablet of a succinic acid health supplement.
The student carries out a titration with potassium hydroxide.
The end point occurs when both acidic protons in succinic acid have been replaced as shown in Equation 19.1.
HOOC(CH2)2COOH + 2KOH → KOOC(CH2)2COOK + 2H2O Equation 19.1
The student uses the following method.
- Stage 1 The student crushes four tablets of the health supplement and dissolves the powdered tablets in distilled water.
- Stage 2 The student makes up the solution from Stage 1 to 250.0 cm3 in a volumetric flask.
- Stage 3 The student titrates 10.0 cm3 portions of the solution obtained in Stage 2 with 0.0600 mol dm–3 potassium hydroxide, using phenolphthalein as the indicator.
The student carries out a trial titration, followed by three further titrations.
The results are shown below.
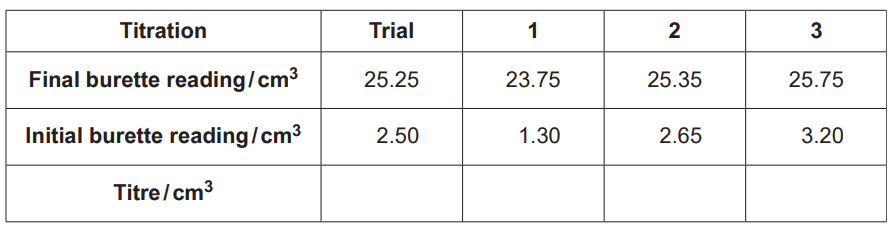
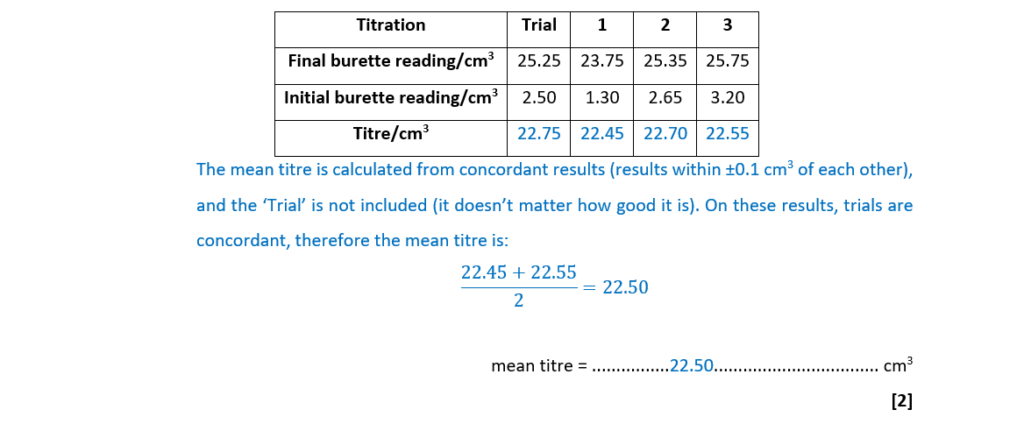
(i)
Complete the table and calculate the mean titre that the student should use for analysing the results.
mean titre = ………………………………………….. cm3 [2]
(ii)
Use the student’s results and Equation 19.1 to calculate the mass, in mg, of succinic acid in one tablet of the health supplement.
Give you answer to 3 significant figures.
mass = …………………………………………… mg [5]


(b)
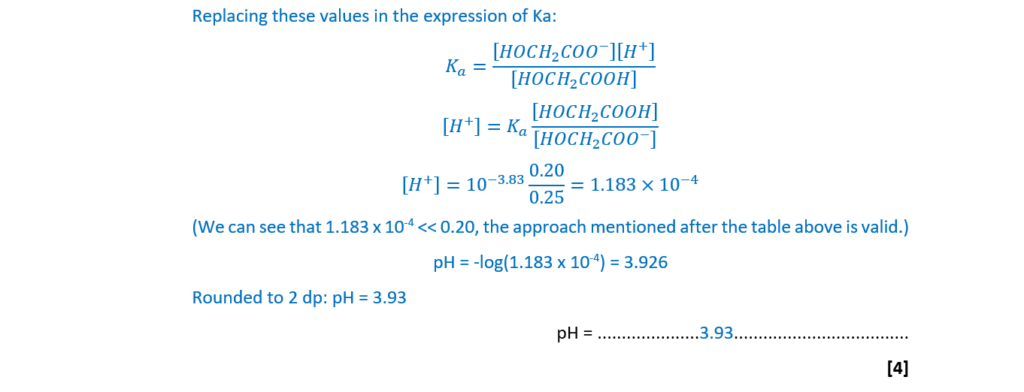
Glycolic acid, HOCH2COOH, (pKa = 3.83) is a weak monobasic acid used in some skincare products.
A buffer solution is prepared by adding 60.0 cm3 of 0.750 mol dm–3 glycolic acid to 40.0 cm3 of 0.625 mol dm–3 potassium hydroxide, KOH.
(i)
Explain why a buffer solution is formed.
[1]

(ii)
Calculate the pH of the buffer solution that has been prepared.
Give your answer to 2 decimal places.
pH = ………………………………………………… [4]
For more exercises about calculations in equilibrium, have a look to Chemical Equilibrium – (jctnotes.com)


(iii)
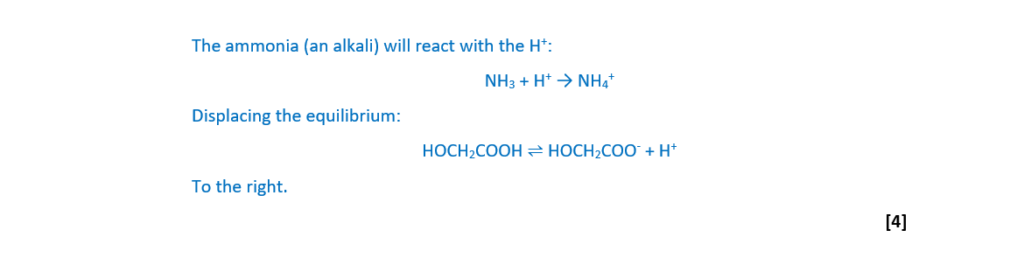
A small amount of aqueous ammonia, NH3(aq), is added to the buffer solution.
Explain, in terms of equilibrium, how the buffer solution would respond to the added NH3(aq).
[4]
20
This question is about equilibria involving hydrogen.
(a)*
Hydrogen is used industrially to manufacture ammonia.
The equilibrium is shown below.
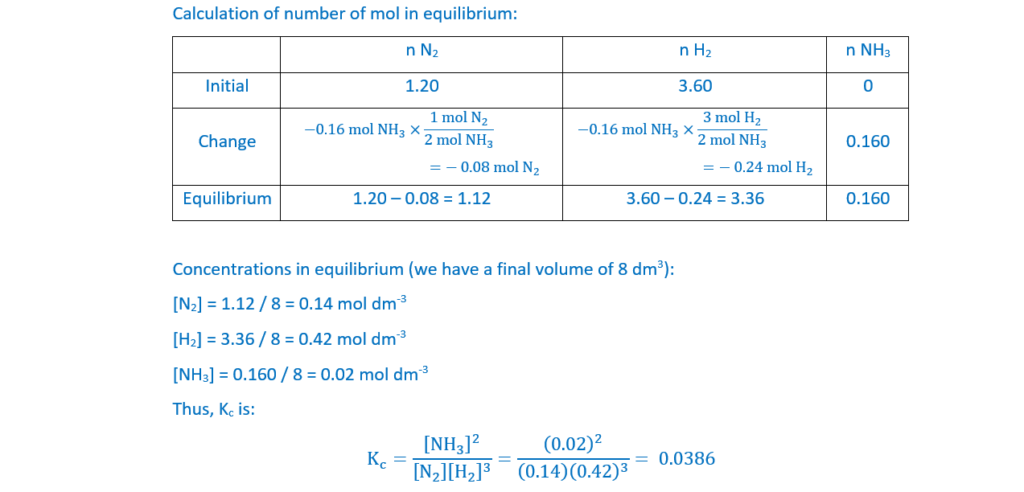
N2(g) + 3H2(g) ⇌ 2NH3(g) ∆H = –92 kJ mol–1 Equilibrium 20.1
1.20 mol N2(g) is mixed with 3.60 mol H2(g) in a 8.00 dm3 container.
The mixture is heated to 550°C with an iron catalyst and allowed to reach equilibrium.
The equilibrium mixture contains 0.160 mol of NH3.
Determine the equilibrium constant Kc for Equilibrium 20.1, and explain why the operational conditions used by industry may be different from those required for a maximum equilibrium yield of ammonia.
[6]


(b)
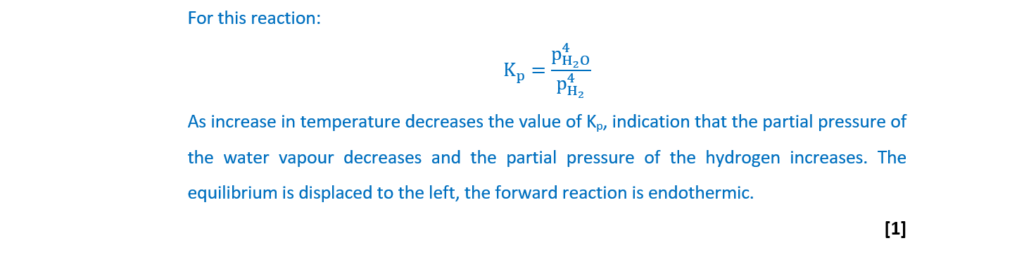
In industry, hydrogen is also used to reduce the iron oxide Fe3O4 as shown in Equilibrium 20.2.
The reaction is carried out at 500°C.
Fe3O4(s) + 4H2(g) ⇌ 3Fe(s) + 4H2O(g) Equilibrium 20.2
(i)
When the temperature is decreased, the value of Kp decreases.
Determine whether the forward reaction is exothermic or endothermic.
Explain your answer.
[1]
(ii)
Two students are discussing the effect of pressure on the equilibrium position of Equilibrium 20.2.
Student 1 says:
“There are more moles of products than reactants, so increasing the pressure will shift the equilibrium to the left hand side.”
Student 2 disagrees.
Determine which student is correct. Justify your answer.
[1]

21
This question is about the reactions of Group 2 metals and their compounds.
(a)
A student adds magnesium to dilute hydrochloric acid in one test tube.
The student adds calcium to dilute hydrochloric acid in a second test tube.
A redox reaction takes place in each test tube.
(i)
Suggest two observations from the student’s experiment that would show that calcium is more reactive than magnesium.
[1]
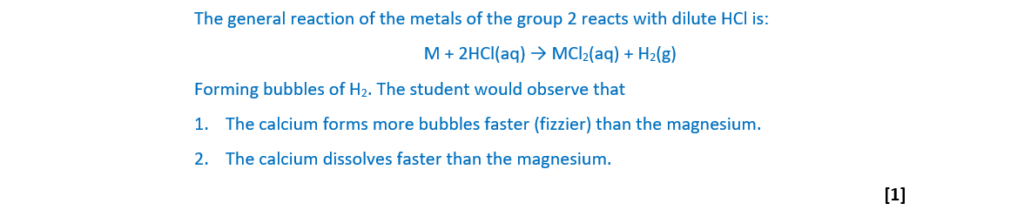
(ii)
Write half-equations for the reaction of magnesium with hydrochloric acid.
Oxidation half-equation: ……………………………………………………………………………………….
Reduction half-equation: ………………………………………………………………………………………
[2]
Oxidation Is Losing electrons and Reduction Is Gaining electrons (mnemotechnic: OIL RIG), we need to find the oxidation state of the elements involved and from there we can find which is oxidized and which one is reduced. For a quick review on Redox equations and some practice exercises, check How to balance redox equations – (jctnotes.com)

(b)
A sample of barium oxide is added to distilled water at 25°C.
A colourless solution forms containing barium hydroxide, Ba(OH)2.
The solution is made up to 250.0 cm3 with distilled water.
The pH of this solution is 13.12.
(i)
Determine the mass of barium oxide that was used.
Give your answer to 3 significant figures.
mass of barium oxide = ………………………………………………. g [5]

(ii)
10 cm3 of dilute sulfuric acid is added to 10 cm3 of the colourless solution of Ba(OH)2.
Write an ionic equation, including state symbols, for the reaction.
[1]

(c)
Limestone and huntite are two calcium minerals.
(i)
A typical sample of limestone contains 95.0% by mass of calcium carbonate, CaCO3.
Fertiliser Z, Ca5NH4(NO3) 11•10H2O (Mr = 1080.5 g mol–1) can be made from limestone.
Calculate the mass, in g, of limestone needed to make 1.50 kg of fertiliser Z.
Give your answer to 3 significant figures.
mass of limestone = ……………………………………………… g [3]

(ii)
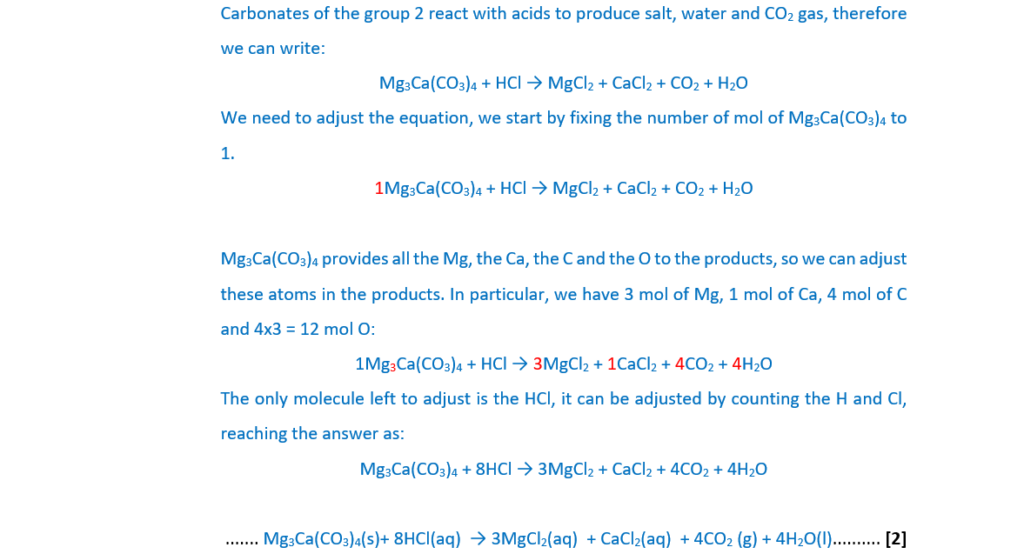
Huntite is a carbonate mineral with the chemical formula Mg3Ca(CO3)4.
Huntite reacts with dilute hydrochloric acid to produce bubbles of a gas and a colourless solution.
Construct the equation for the reaction. Include state symbols.
[2]
For further explanation and practice exercises to balance chemical equations, check How to balance chemical equations – (jctnotes.com)
22
This question is about reactions of transition metal compounds.
(a)
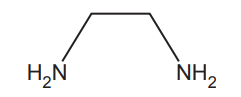
Ethane-1,2-diamine, H2NCH2CH2NH2, is a bidentate ligand.
The structure of ethane-1,2-diamine is shown below.
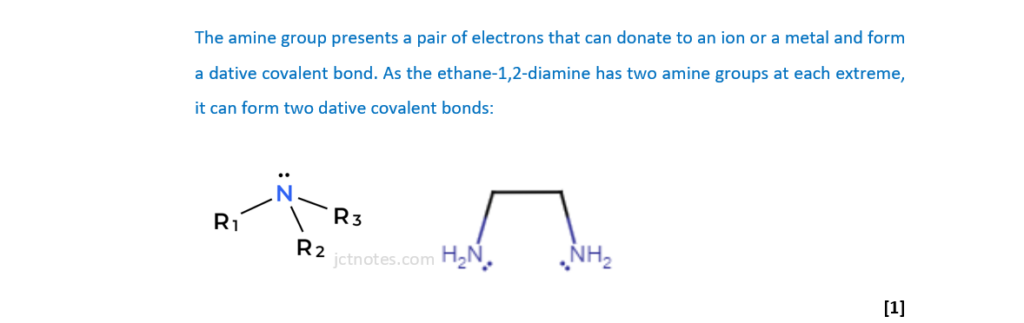
(i)
Explain why ethane-1,2-diamine can act as a bidentate ligand.
[1]
(ii)
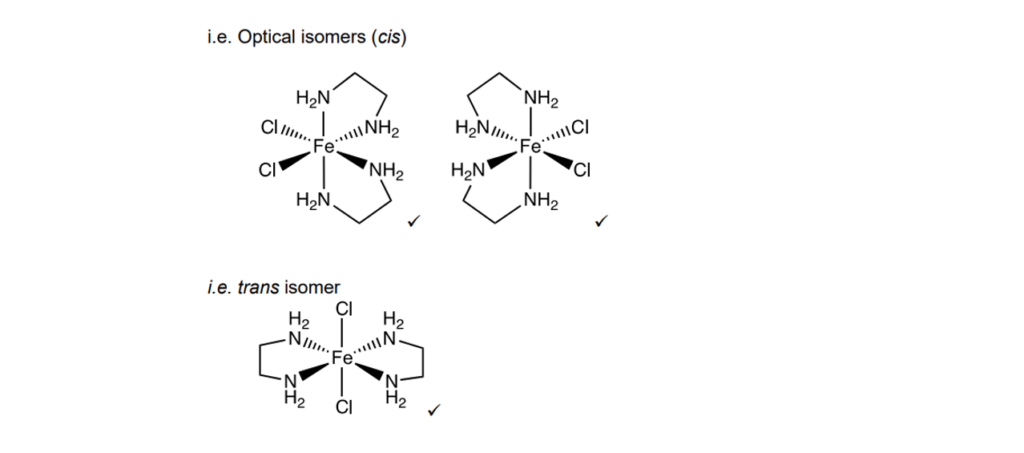
The iron(III) ion, Fe3+, forms a complex ion A with two ethane-1,2-diamine ligands and two chloride ligands.
Complex ion A has cis and trans stereoisomers.
One of these stereoisomers exists as optical isomers.
Determine the empirical formula, with charge, of complex ion A and draw the 3-D structures of the three stereoisomers.
Empirical formula with charge ………………………..



(b)
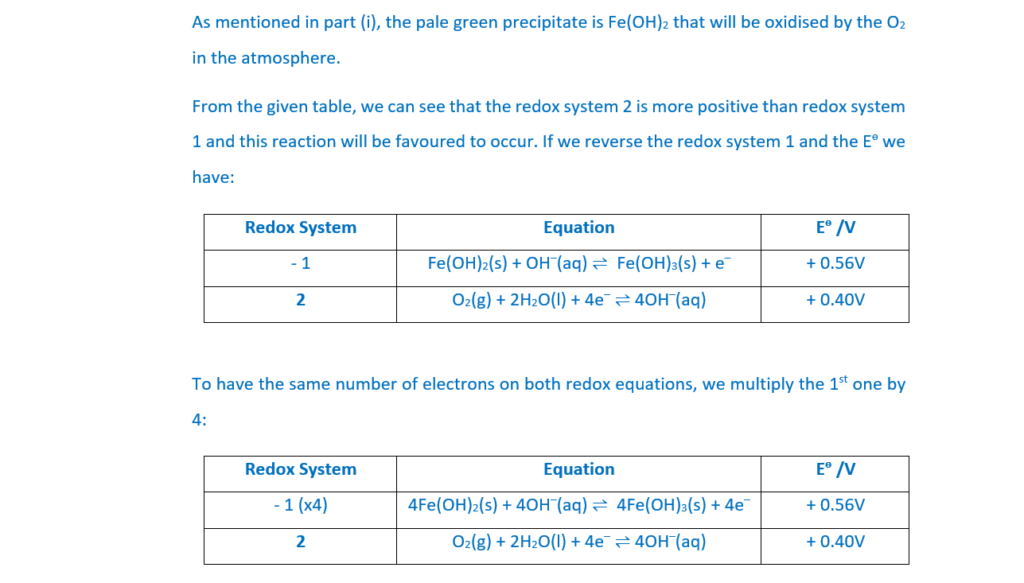
Aqueous sodium hydroxide is added to an aqueous solution of iron(II) sulfate.
A pale green precipitate forms which turns brown when left to stand in air
(i)
Write an ionic equation for the formation of the pale green precipitate.
Note, looking at part (ii) of this question give a clue of what is going on.
The green precipitate is Fe(OH)2. Oxidation with air will produce brown Fe(OH)3 (A link to the colour of precipitates can be found in A Level Chemistry A – Colours of inorganic ions and complexes (ocr.org.uk)). The ionic equation is:
……………………………………Fe2+ + 2OH– → Fe(OH)2 ………………………….. [1]
(ii)
Use the information below to explain why the pale green precipitate turns brown when left to stand in air and construct an equation for the reaction which occurs.


(c)*
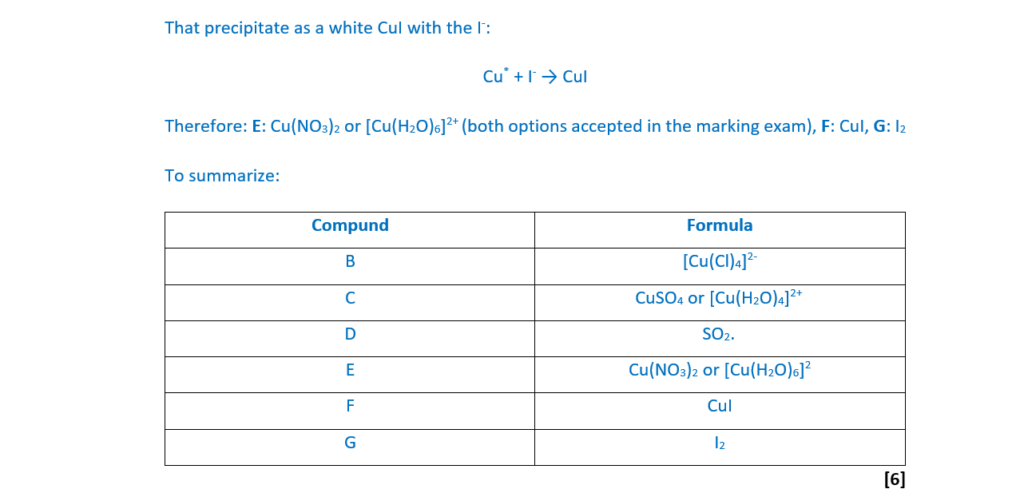
This question is about copper and copper compounds.
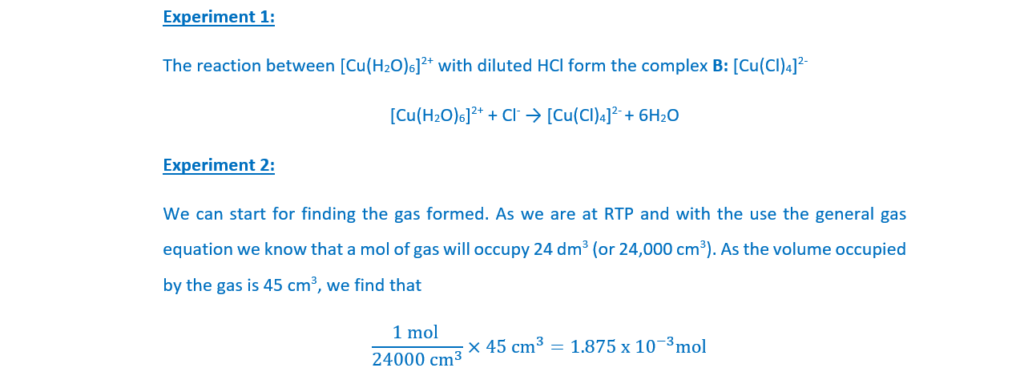
Experiment 1
Hydrochloric acid, HCl(aq), is added to an aqueous solution containing [Cu(H2O)6]2+ complex ions.
A yellow-green solution forms containing complex ion B.
Experiment 2
A piece of copper metal is heated with concentrated sulfuric acid.
A reaction takes place forming a pale blue solution C and 45 cm3 of a gas D, measured at RTP. The mass of gas D is 0.12g.
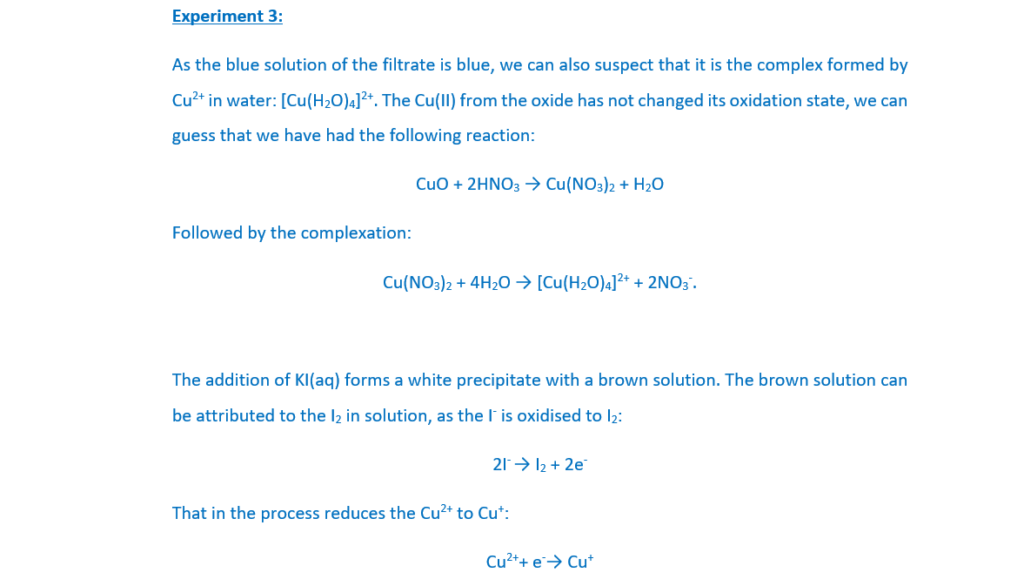
Experiment 3
An excess of copper(II) oxide is heated with dilute nitric acid. The resulting mixture is filtered. The filtrate is a blue solution E.
Aqueous potassium iodide, KI(aq), is added to the blue solution E.
A white precipitate F and a brown solution G form.
Determine the formulae of B–G.
Construct equations for the reactions taking place, include any changes in oxidation number, and show your working where appropriate.
.
[6]
Most of the information for this problem can be found in A Level Chemistry A – Colours of inorganic ions and complexes (ocr.org.uk). Try to memorize these colours for the exam.

END OF QUESTION PAPER
External links:
- Paper: A Level Chemistry A H432/01 June 2022 (ocr.org.uk)
- Post exam correction: H432-01 Post Exam Correction Jun22 (ocr.org.uk)
- Mark scheme: Mark Scheme H432/01 Periodic table, elements and physical chemistry June 2022 (ocr.org.uk)
- OCR data sheet for the exam: AS GCE (H032) A GCE (H432) Data Sheet for Chemistry A (ocr.org.uk)
- OCR colours of inorganic ions and complexes: A Level Chemistry A – Colours of inorganic ions and complexes (ocr.org.uk)
