If you find this useful, please leave a comment at the end of the page.
I have created a video with this content. You can see it in: OCR 2022 A Level Chemistry Paper 2, synthesis and analytical techniques. – YouTube

SECTION A
You should spend a maximum of 20 minutes on this section.
Write your answer to each question in the box provided.
1
Which statement is correct for the different rates of hydrolysis of RCl and RBr?
A RBr is hydrolysed faster because Cl is more electronegative than Br.
B RBr is hydrolysed faster because the C–Cl bond enthalpy is greater than C–Br.
C RCl is hydrolysed faster because Cl is more electronegative than Br.
D RCl is hydrolysed faster because the C–Br bond enthalpy is greater than C–Cl.
Your answer
[1]


3
3. What is the number of sigma bonds in a molecule of methylbenzene?
A 7
B 10
C 12
D 15
Your answer
[1]

4
The skeletal formula of an organic compound is shown below.
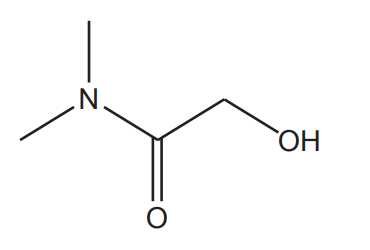
Which functional groups are present?
A amide and alcohol
B amide and carboxylic acid
C amine and carboxylic acid
D amine, ketone and alcohol
Your answer
[1]

5
The structure of a drug is shown below:
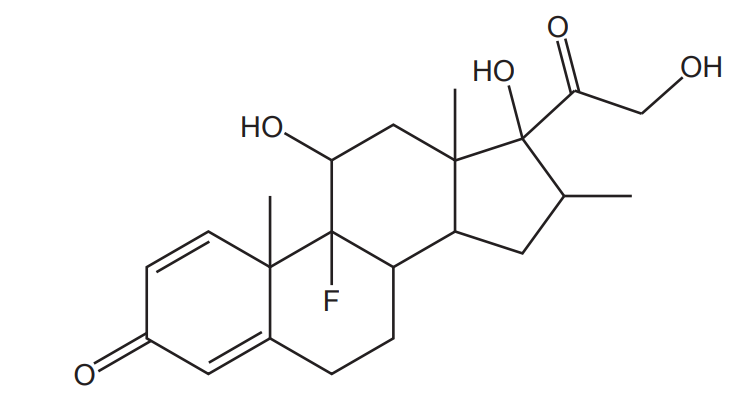
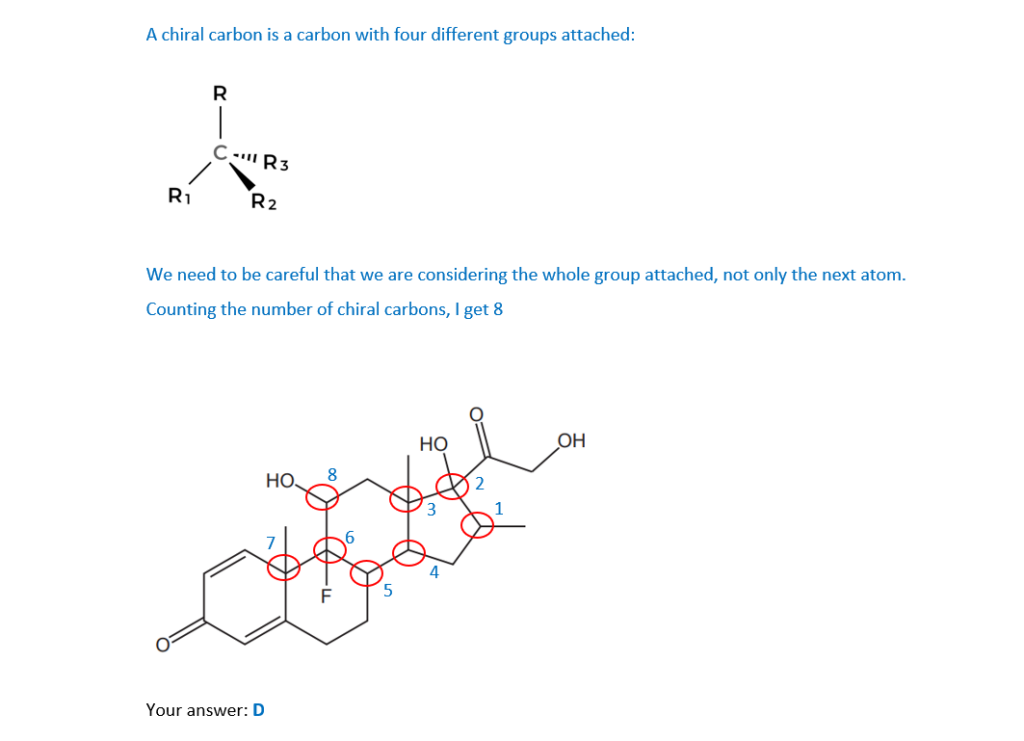
How many chiral carbon atoms are there in a molecule of the drug?
A 5
B 6
C 7
D 8
Your answer
[1]
6
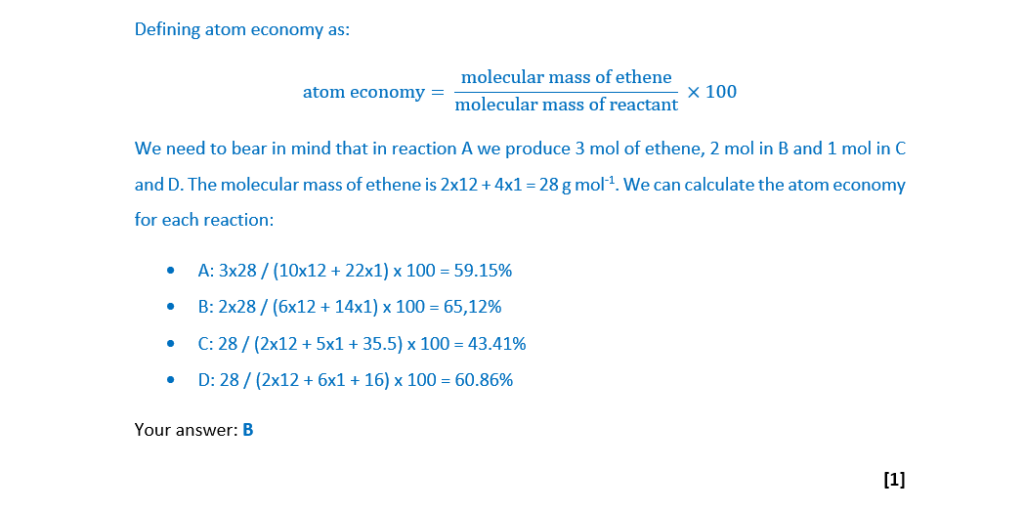
Which process has the highest atom economy for preparing ethene, C2H4?
In each process, assume that ethene is the only product that is used.
A C10H22 → 3C2H4 + C4H10
B C6H14 → 2C2H4 + C2H6
C C2H5Cl → C2H4 + HCl
D C2H5OH → C2H4 + H2O
Your answer
[1]


8
The structure of the painkiller, paracetamol, is shown below.
A tablet contains 3.31 × 10–3 mol of paracetamol. What is the mass of paracetamol in the tablet?
A 493mg
B 497mg
C 500mg
D 506 mg
Your answer
[1]
The formula of the paracetamol can be written as C8H9O2N, with a molecular mass: 8×12 + 9×1 +2×16 + 14 = 151 g mol-1.
With the molecular mass, we can find that 3.31 × 10–3 mol of paracetamol is:
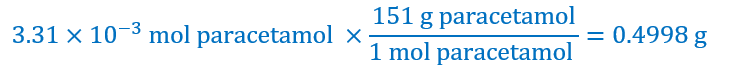
Expressed in mg is 500 mg.
Your answer: C
[1]
9
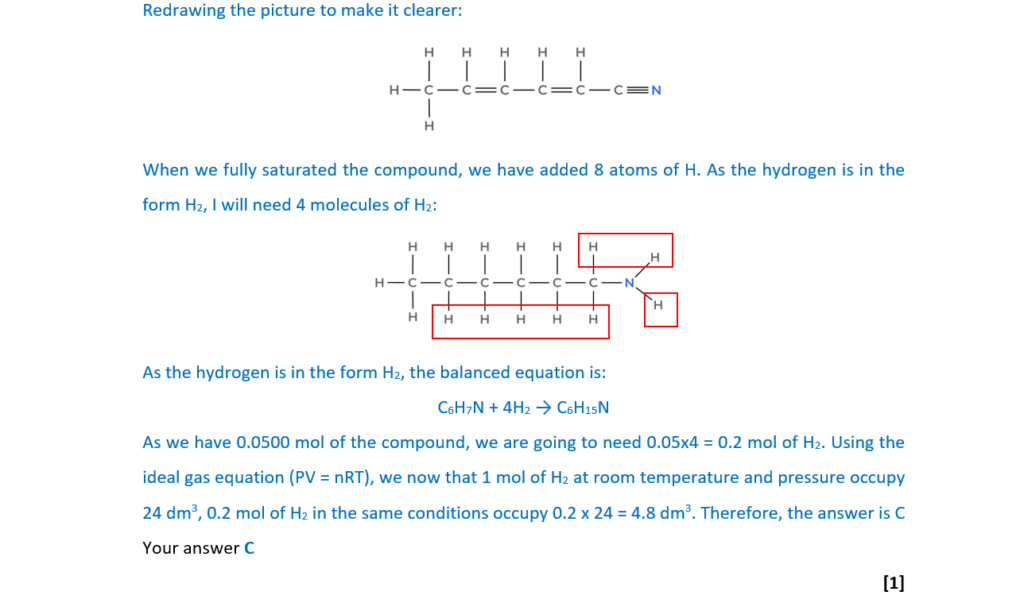
The compound below reacts with hydrogen gas to form a saturated compound.
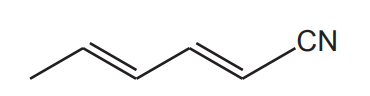
What is the volume of hydrogen, measured at room temperature and pressure, that reacts with 0.0500 mol of the compound?
A 2.40 dm3
B 3.60 dm3
C 4.80 dm3
D 6.00 dm3
Your answer
[1]
10
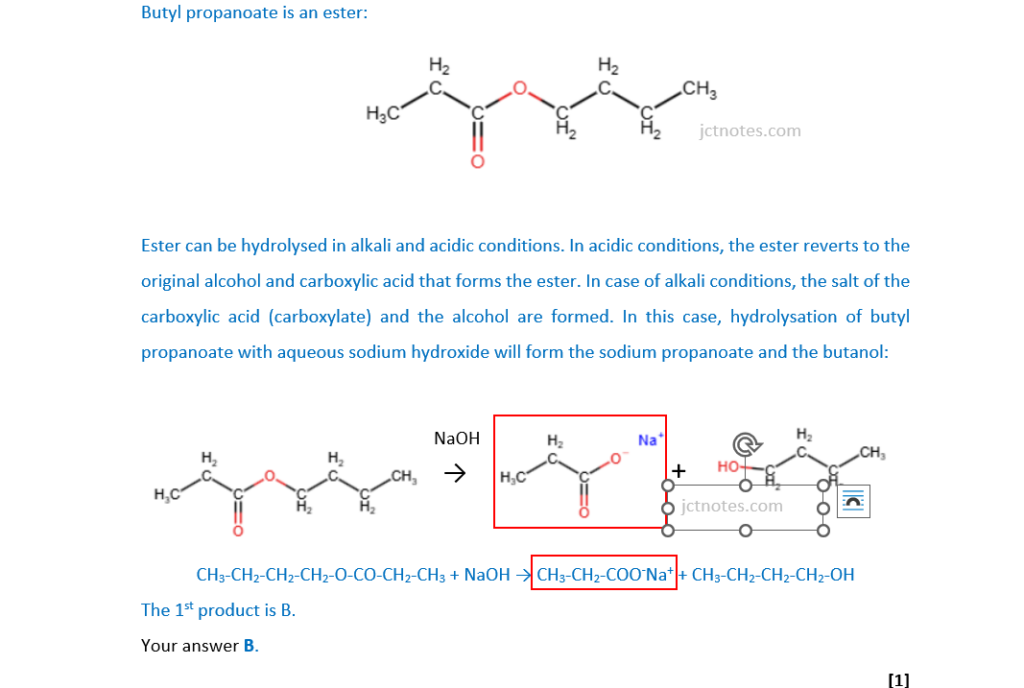
Butyl propanoate is hydrolysed by aqueous sodium hydroxide.
Which compound is one of the products of this hydrolysis?
A C3H7ONa
B C3H5O2Na
C C4H9ONa
D C4H7O2Na
Your answer
[1]
11
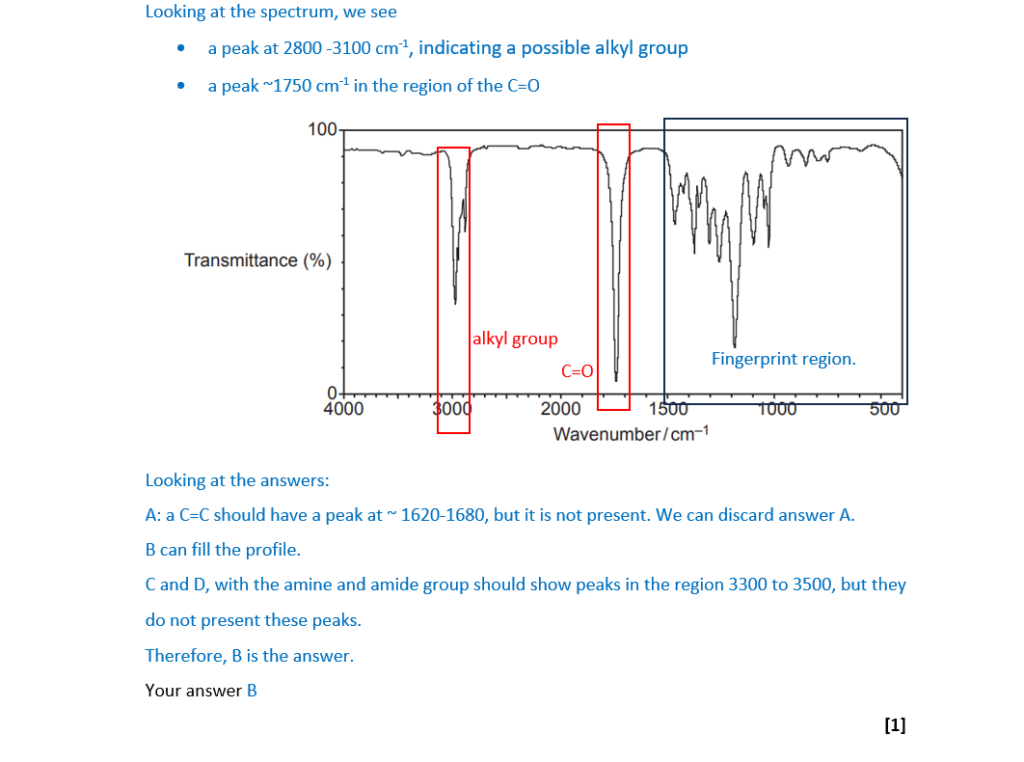
The infrared spectrum of an organic compound is shown below.

Which compound could have produced this spectrum?
A H2C=CHCH2CH2OH
B CH3COOCH2CH3
C H2NCH2COOCH3
D (CH3)2CHCONH2
Your answer
[1]
12
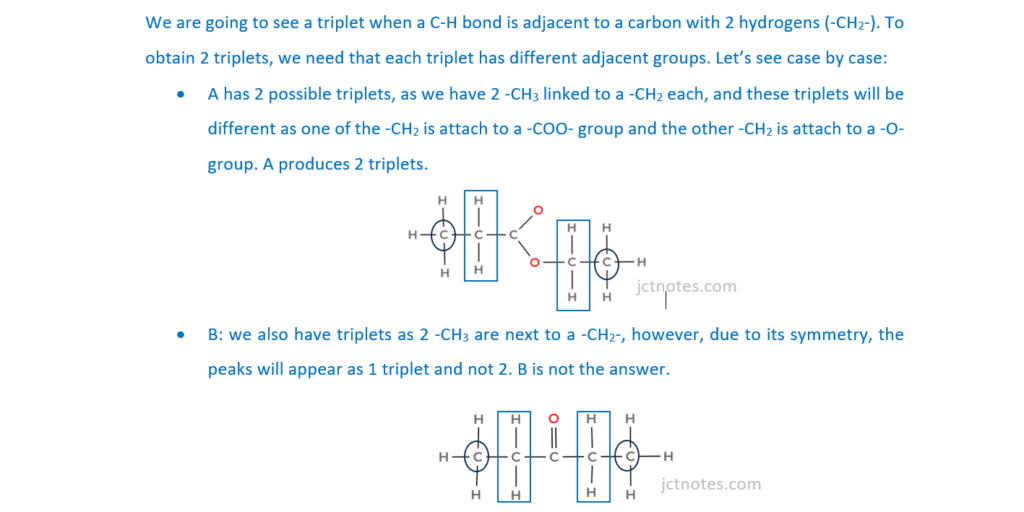
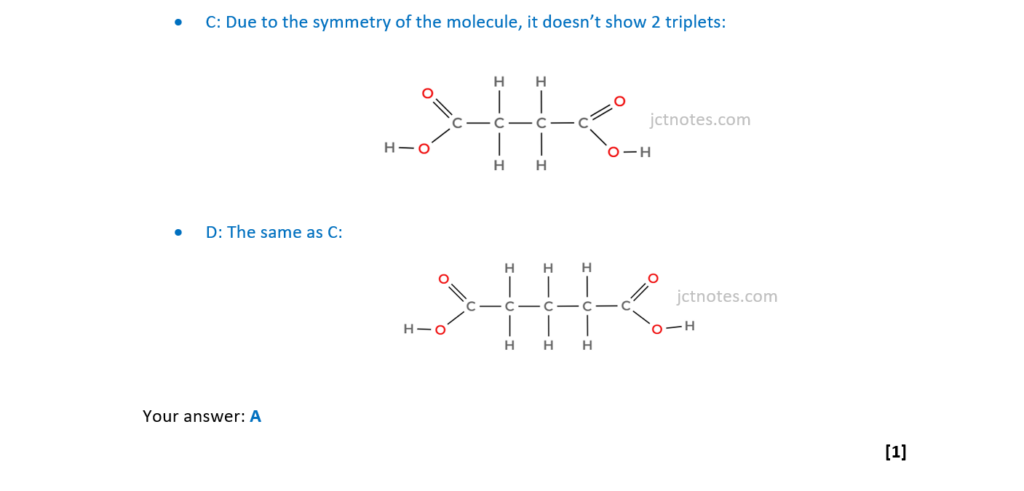
Which compound produces two triplets in its 1H NMR spectrum?
A CH3CH2COOCH2CH3
B CH3CH2COCH2CH3
C HOOCCH2CH2COOH
D HOOCCH2CH2CH2COOH
Your answer
[1]
13
Which equation(s) could be part of the propagation step in the radical substitution of C5H12 to form C5H11Cl?
1 C5H11• + Cl2 → C5H11Cl + Cl•
2 C5H12 + Cl• → C5H11Cl + H•
3 C5H11• + Cl• → C5H11Cl
A 1, 2 and 3
B Only 1 and 2
C Only 2 and 3
D Only 1
Your answer
[1]


14.
Which species could react as a nucleophile?
1 NH3
2 OH–
3 CH3NH2
A 1, 2 and 3
B Only 1 and 2
C Only 2 and 3
D Only 1
Your answer
[1]

15
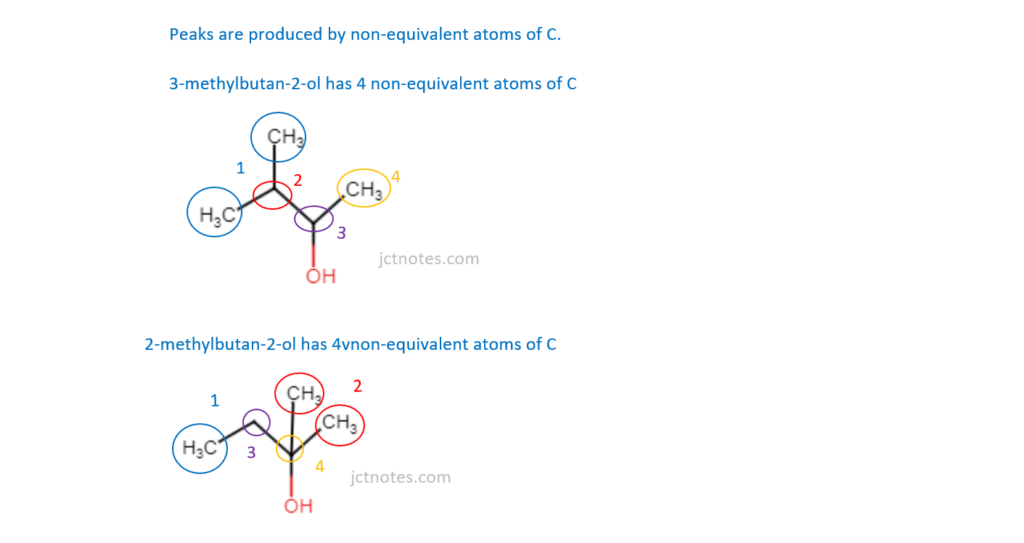
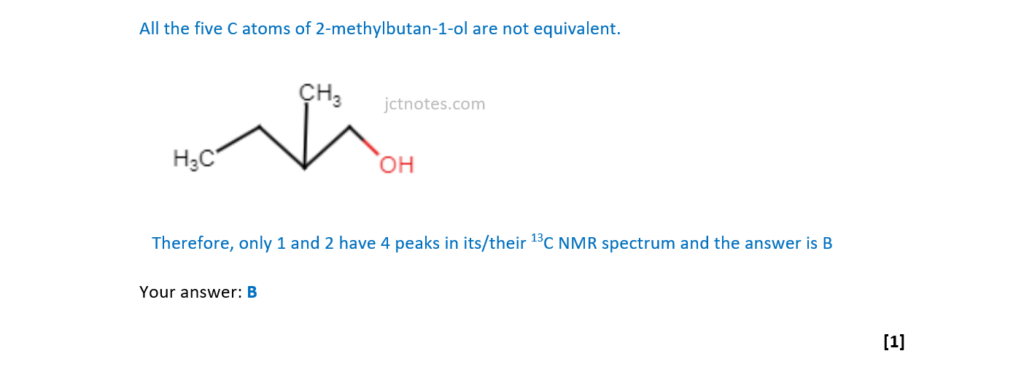
Which isomer(s) of C5H12O has/have 4 peaks in its/their 13C NMR spectrum?
1 3-methylbutan-2-ol
2 2-methylbutan-2-ol
3 2-methylbutan-1-ol
A 1, 2 and 3
B Only 1 and 2
C Only 2 and 3
D Only 1
Your answer
[1]
External links:
- Paper: H432-02_G_Jun22_301077.indd (ocr.org.uk)
- Mark scheme: Mark Scheme H432/02 Synthesis and analytical techniques June 2022 (ocr.org.uk)
- OCR data sheet for the exam: AS GCE (H032) A GCE (H432) Data Sheet for Chemistry A (ocr.org.uk)
- OCR reaction pathways OCR A Level Chemistry A – Topic Exploration Pack – Reaction pathways
