If you find this useful, please leave a comment at the end of the page.
I have created a video with this content. You can see it in: OCR 2022 A Level Chemistry Paper 2, synthesis and analytical techniques. – YouTube

SECTION B
16
This question is about unsaturated hydrocarbons.
(a)
The unsaturated hydrocarbon A, shown below, is reacted with bromine.

(i)
What is the systematic name of hydrocarbon A?
3-methylhex-2-ene
[1]
(ii)
Outline the mechanism for the reaction of hydrocarbon A with bromine. The structure of hydrocarbon A has been provided. Include curly arrows and relevant dipoles.
[3]
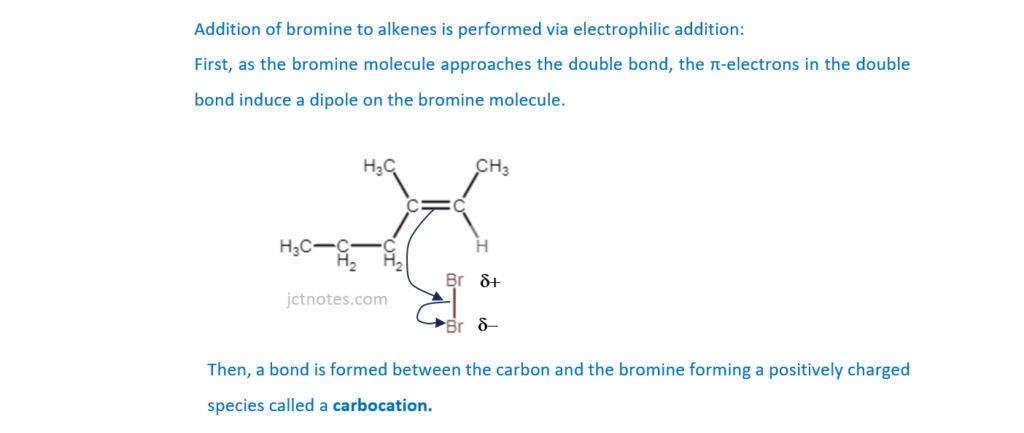
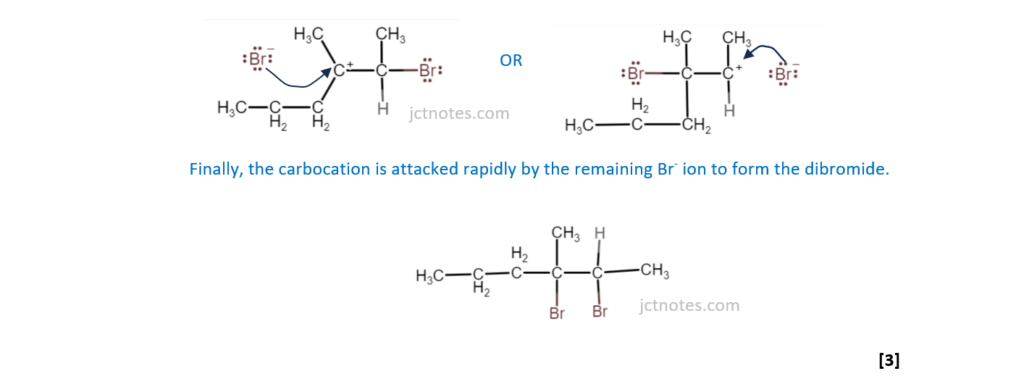
(b)
Compounds B and C are branched hydrocarbons that are structural isomers of C6H12.
Compounds B and C both have stereoisomers.
• Compound B has cis and trans isomers but does not have optical isomers.
• Compound C has optical isomers but does not have cis and trans isomers.
(i)
What is meant by the term structural isomers?
Structural isomers are compounds with the same molecular formula but different structural formulae.
[1]
(ii)
What is meant by the term stereoisomers?
Stereoisomers are compounds whose molecules have the same atoms bonded to each other but with different arrangements of the atoms in space.
[1]
(iii)
Draw structures for the cis and trans isomers of the branched hydrocarbon B.
[1]
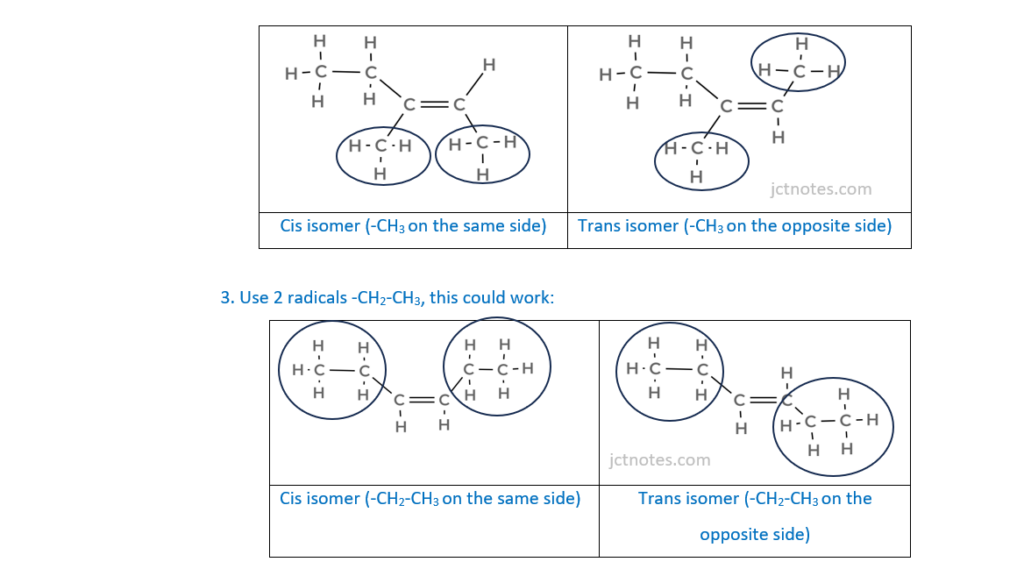
[2]
(iv)
Draw 3D structures for the optical isomers of compound C.
[2]
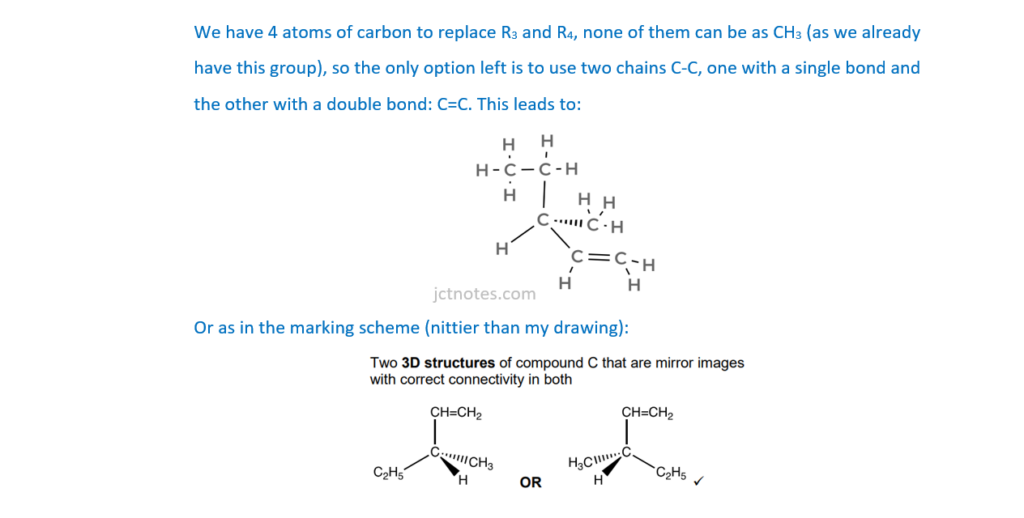
[2]
(v)
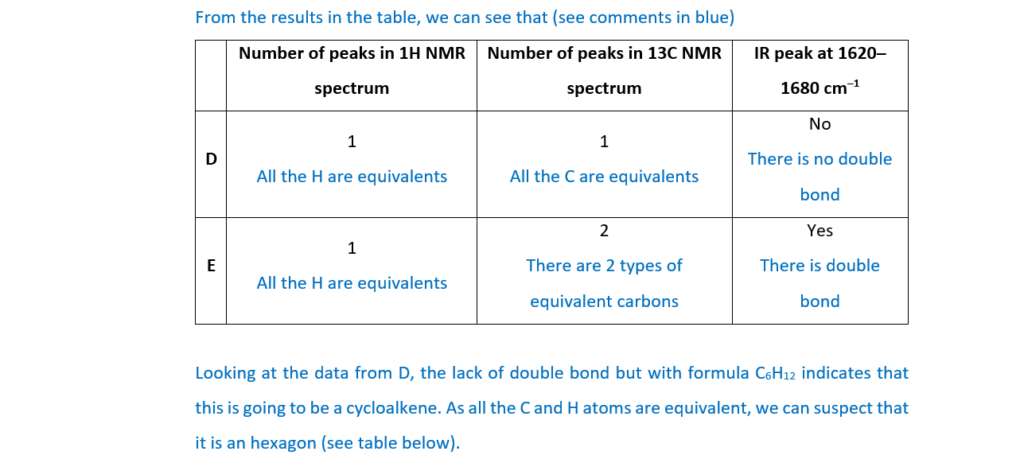
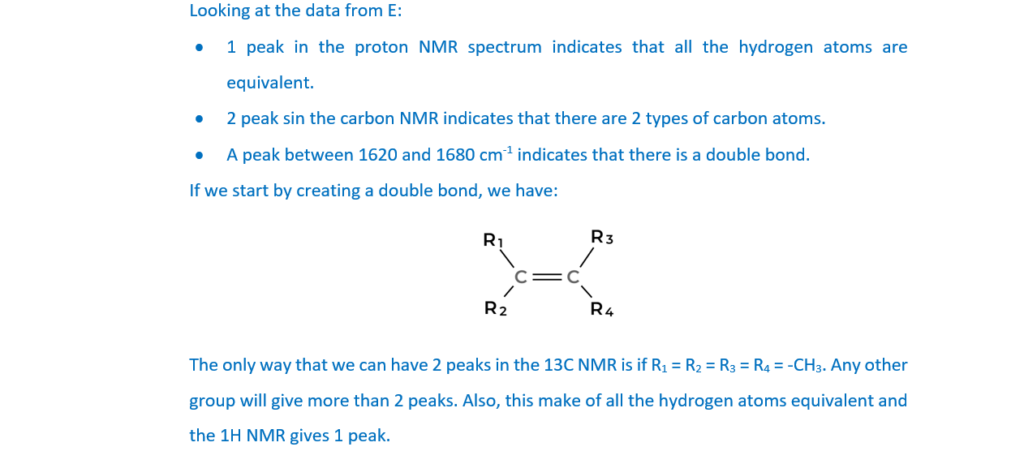
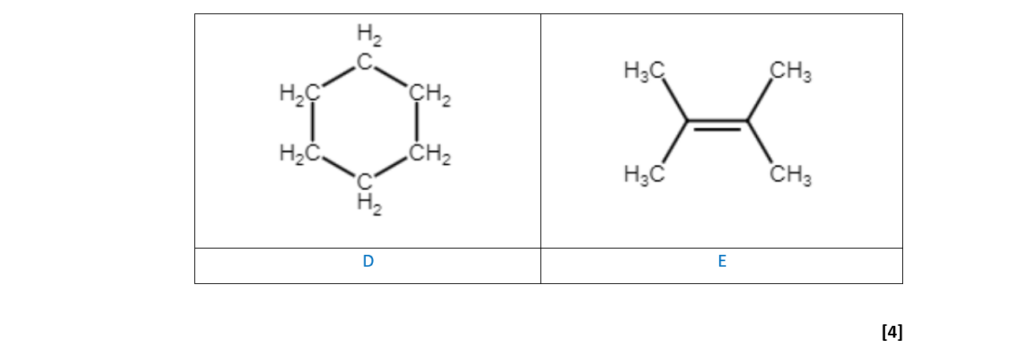
Compounds D and E are two more structural isomers of C6H12.
Compounds D and E do not show stereoisomerism.
Table 16.1 shows NMR and infrared (IR) spectral data for D and E.
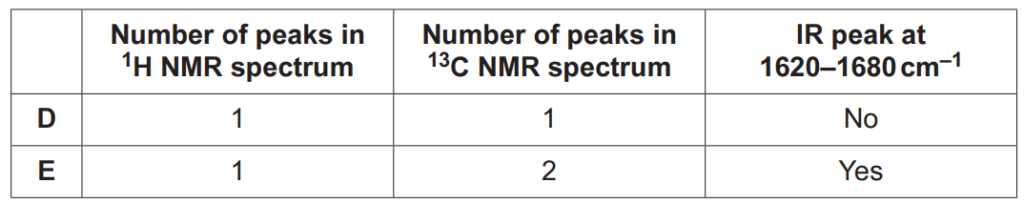
Draw the structures of D and E and explain how the spectral data in Table 16.1 provides evidence for the structures.
[4]
(c)
‘Ozonolysis’ is used in organic synthesis. Ozone breaks C=C bonds to form carbonyl compounds.
For example, the complete ozonolysis of methylbut-2-ene is shown below.
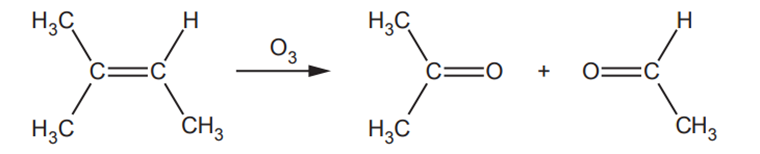
(i)
Draw the structures of the products you would expect from the ozonolysis of the two compounds below.
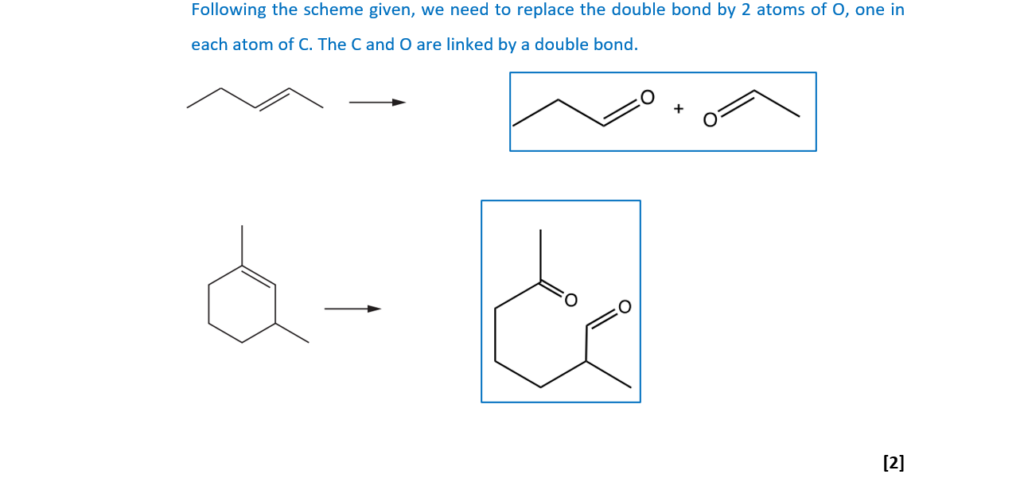
(iii)
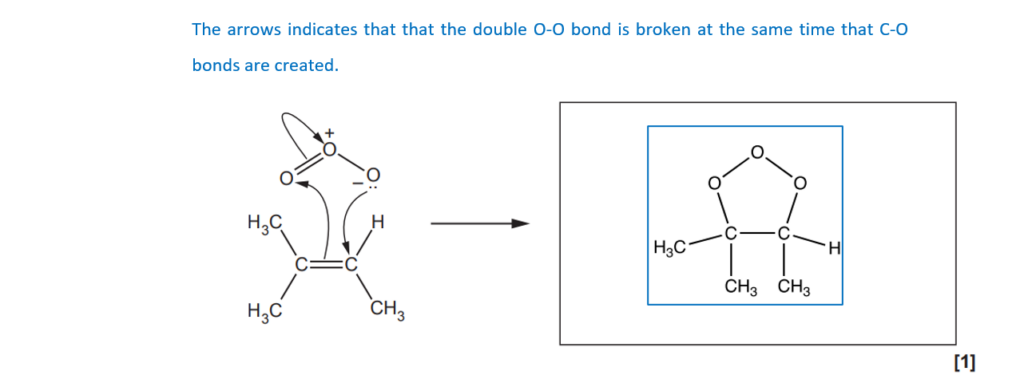
The mechanism for ozonolysis takes place in several steps.
The curly arrows in the first step in the ozonolysis of methylbut-2-ene are shown below.
In the box, draw the structure(s) for the product(s) of this step.
[1]
17
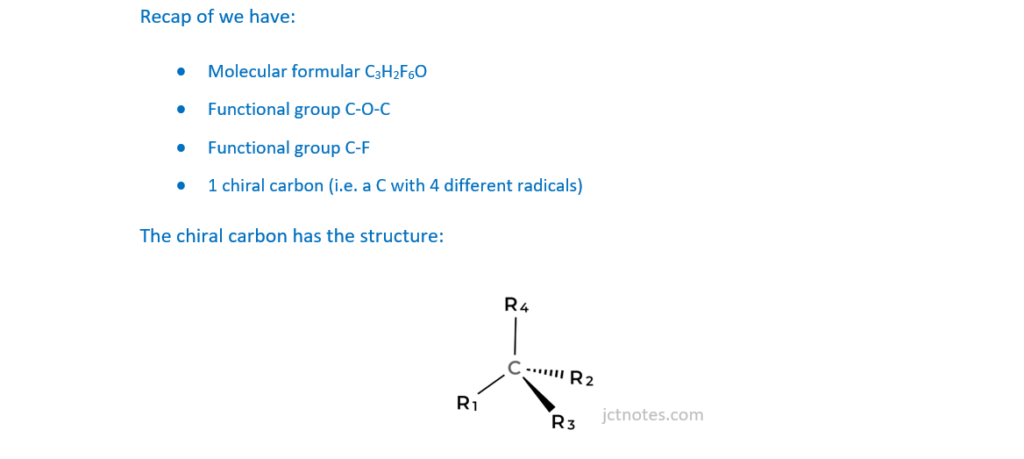
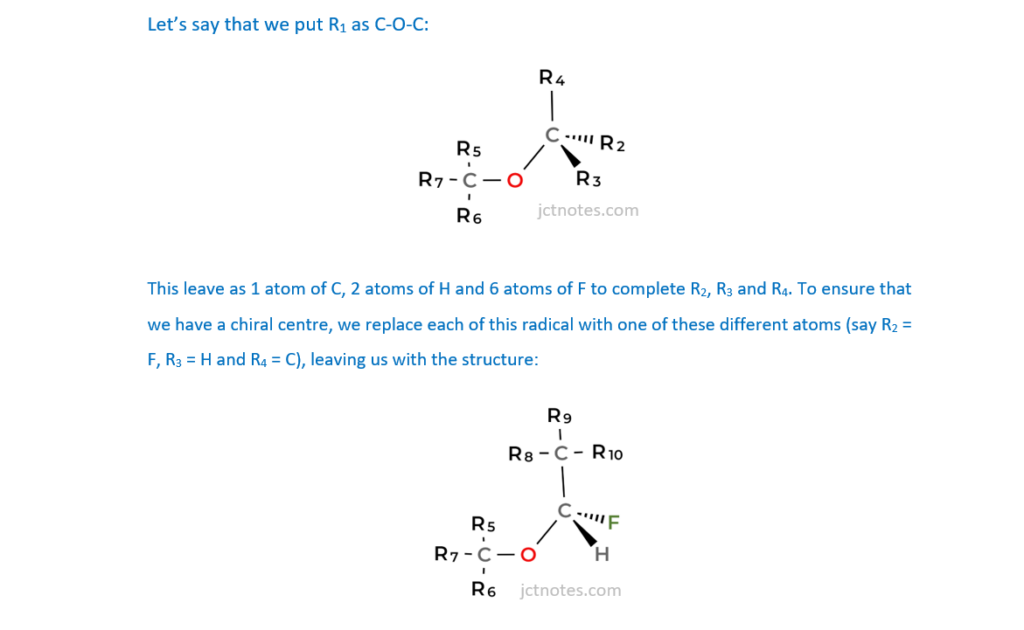
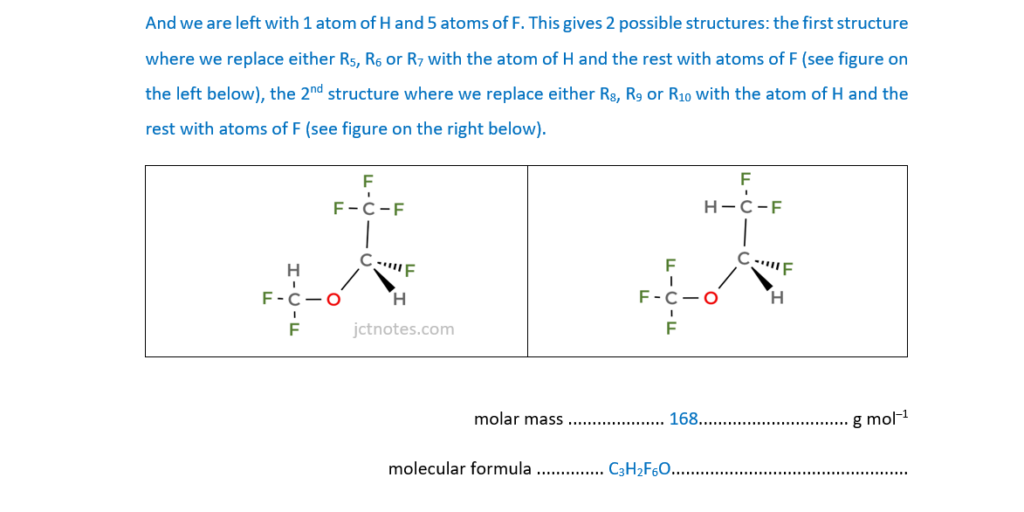
This question is about an analysis of an unknown organic Compound X.
Some properties of Compound X are shown in the table.
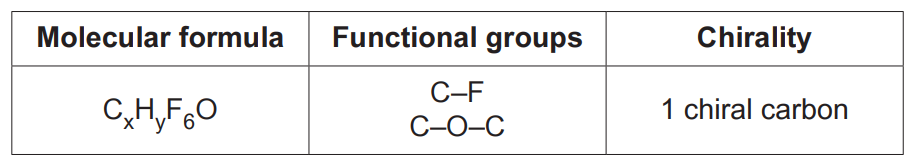
At a pressure of 1.07 × 105Pa at 30°C, 1.327g of Compound X is a gas with a volume of 186 cm3.
Determine the molar mass of Compound X and its molecular formula.
Draw a possible structure for a molecule of Compound X.
molar mass …………………………………………… g mol–1
molecular formula ………………………………………………………
Structure of Compound X
[6]



18
This question is about carbonyl compounds.
(a)
(i)
Describe a chemical test to confirm the presence of a carbonyl group.
How could the product of this test be used to identify the carbonyl compound?
Your answer should not include spectroscopy.
This a very standard test to identify carbonyl groups (aldehyde and ketones). To obtain the 3 marks, cover the 3 following points:
- Addition of a solution of 2,4-DNPH will produce an orange precipitate.
- The precipitate can be purified by recrystallisation.
- Melting point of the crystals obtained can be used to identify the compound by comparing to known values in database.
[3]
(ii)
Describe a chemical test that would identify whether a carbonyl compound is an aldehyde.
Your answer should include the reagent and observations.
Addition of Tollens’ reagent (an aqueous solution of silver nitrate in excess ammonia solution) will produce a grey precipitate of silver, or a silver mirror is formed on the test tube.
An alternative method accepted in the mark scheme is the addition of acidified dichromate(VI) will be turned green.
[1]
(b)
A student is provided with two unknown carbonyl compounds, F and G.
The compounds are analysed and found to have identical percentage compositions by mass:
C: 62.07%; H: 10.34%; O: 27.59%
The mass spectra of the two compounds are shown below.
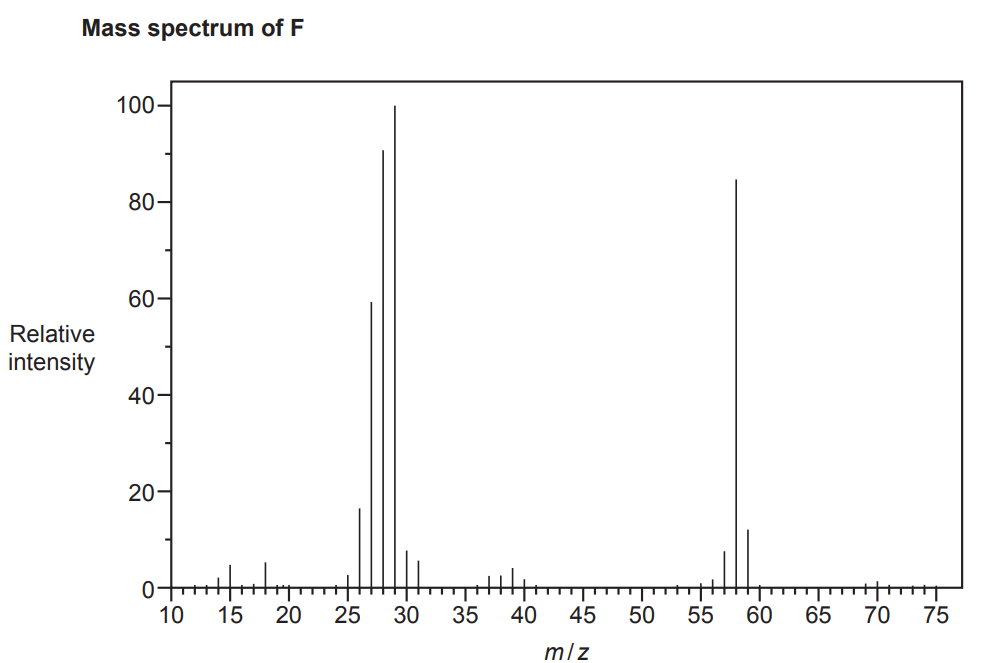
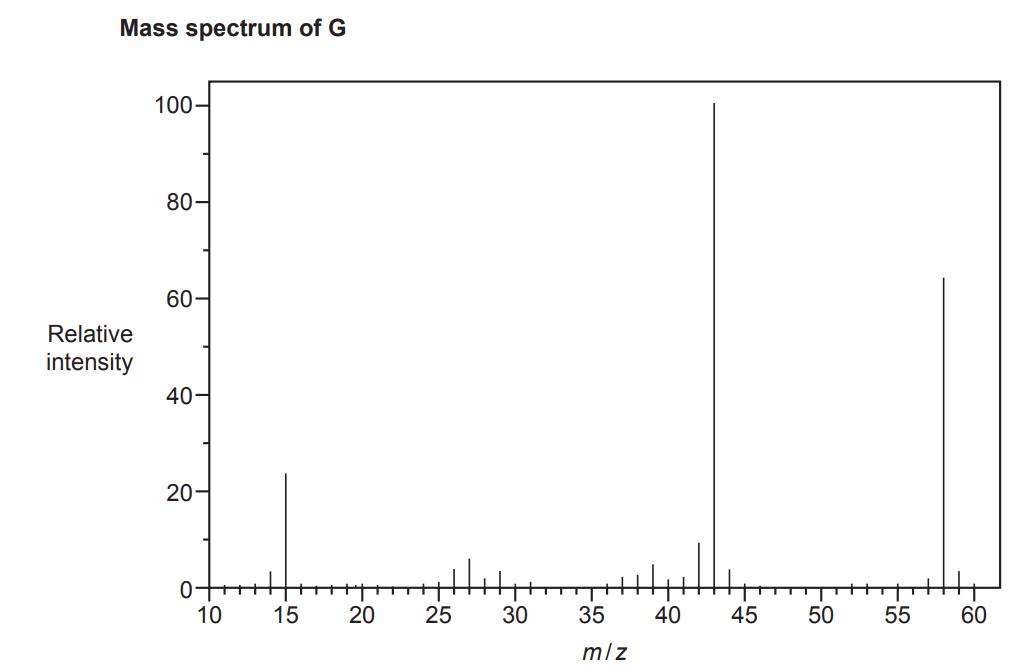
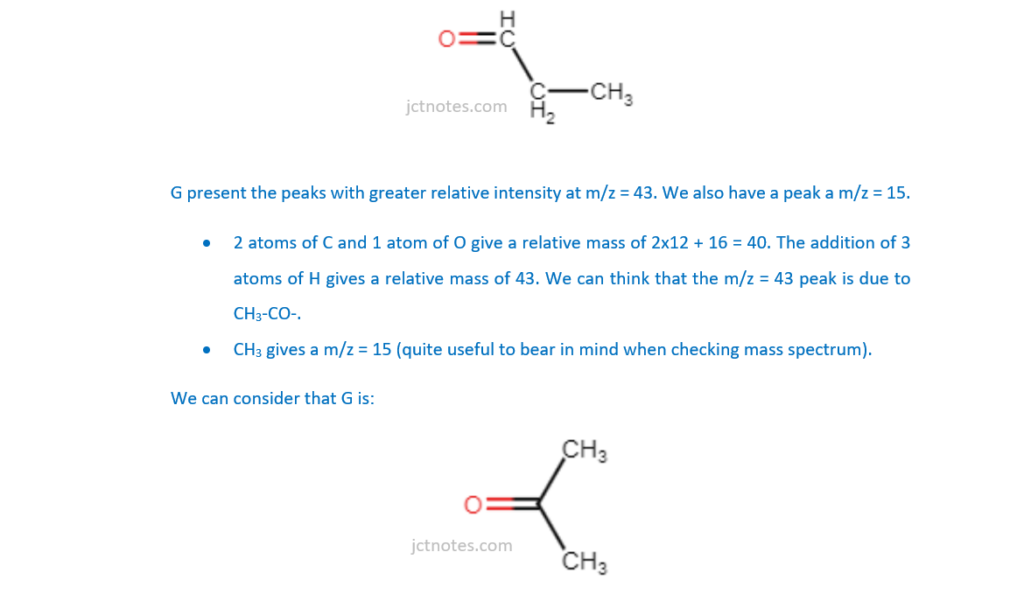
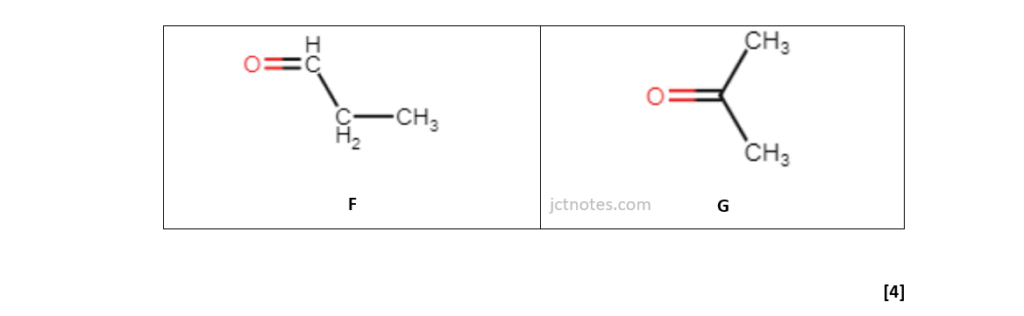
Use the results to identify the structures of the two compounds.
Include relevant peaks present in the mass spectrum of each compound.
[4]
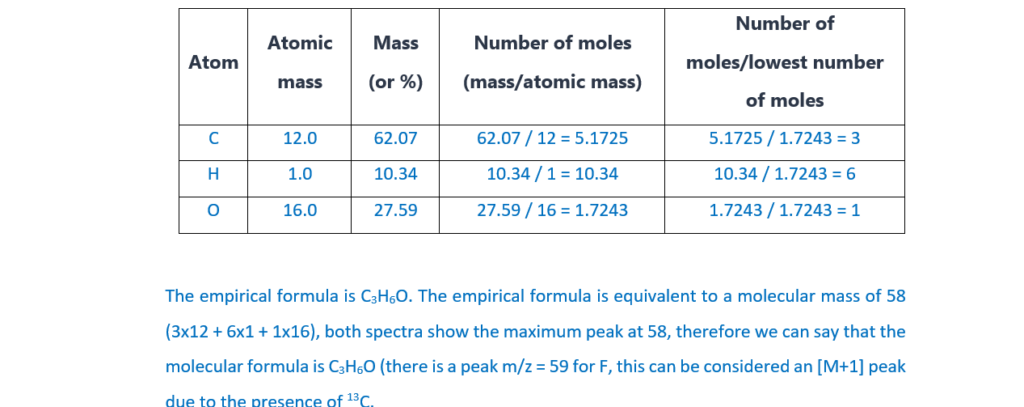
We start by calculating the empirical formula of the compounds (for an explanation about how to calculate empirical formulas and examples, see the following link: Formulas and formula masses – (jctnotes.com))

(c)
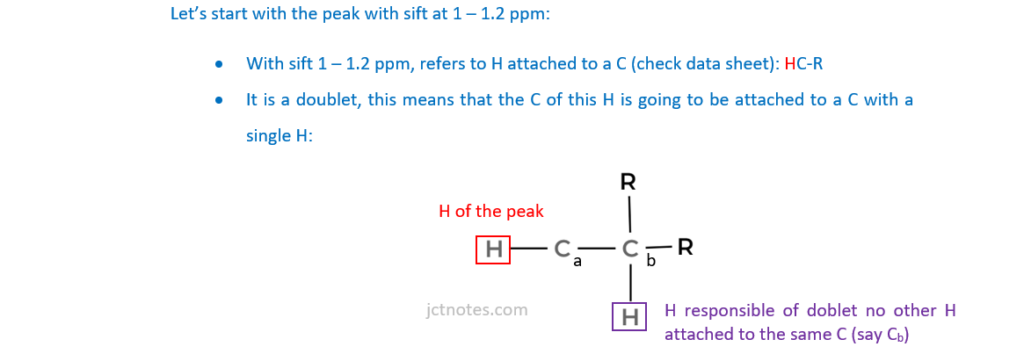
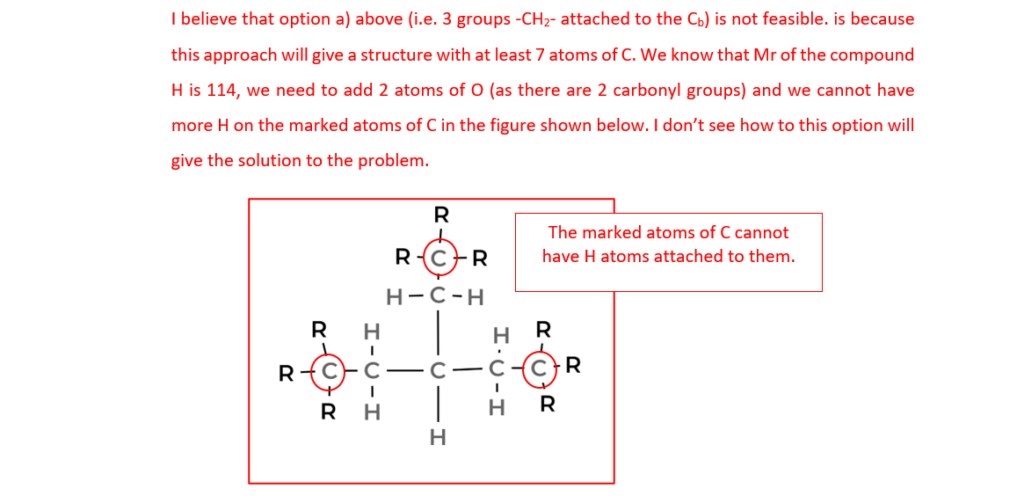
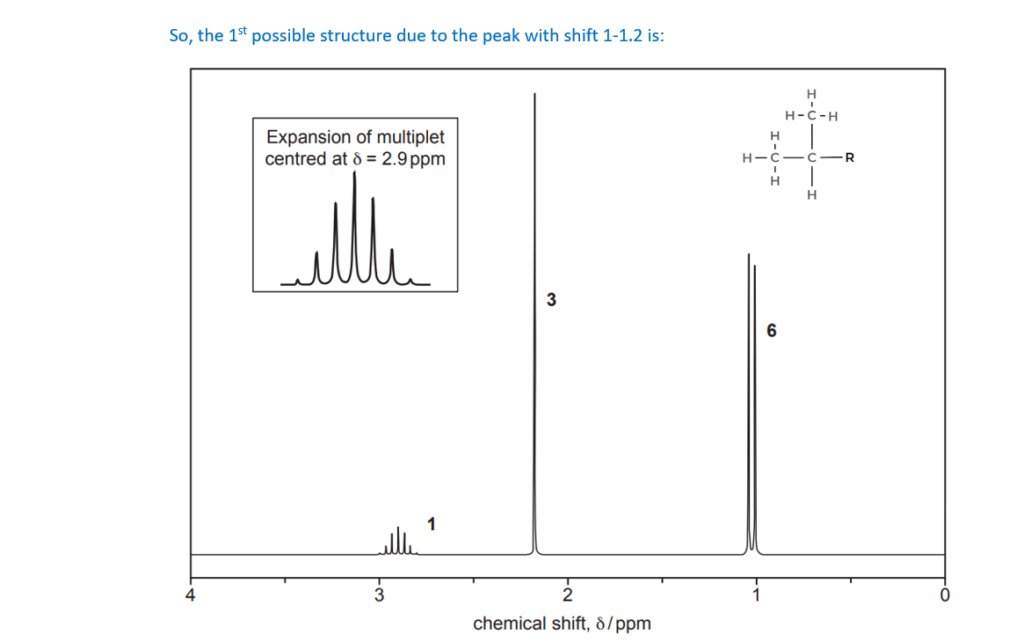
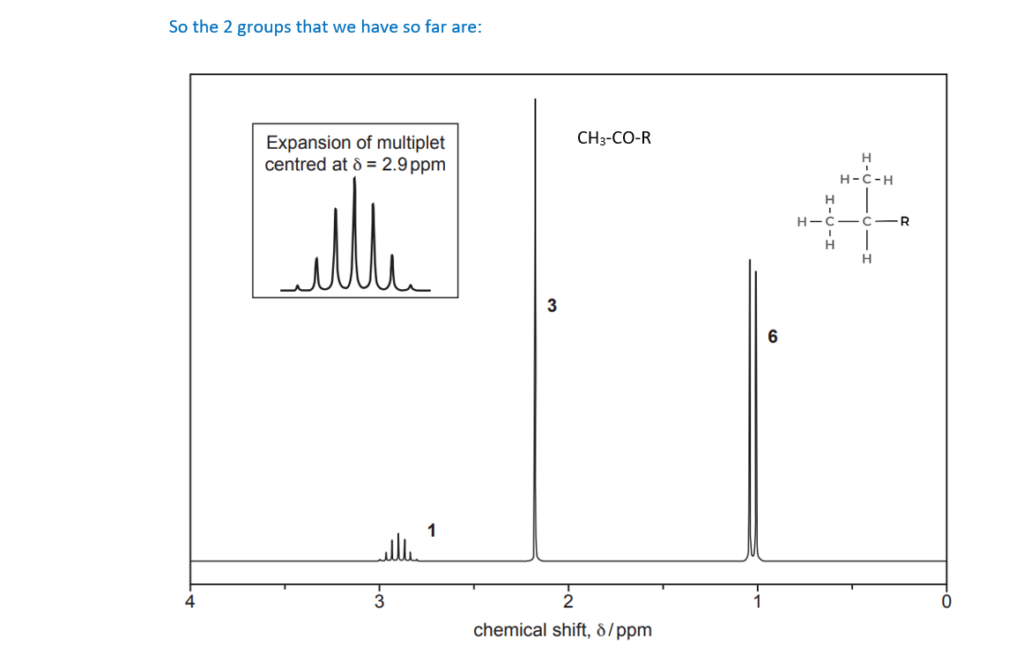
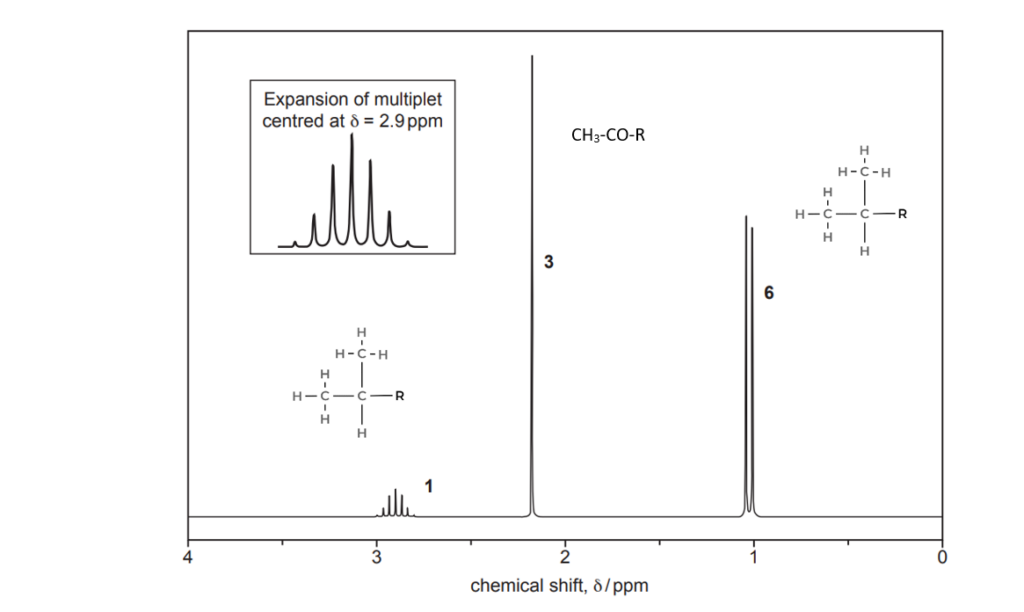
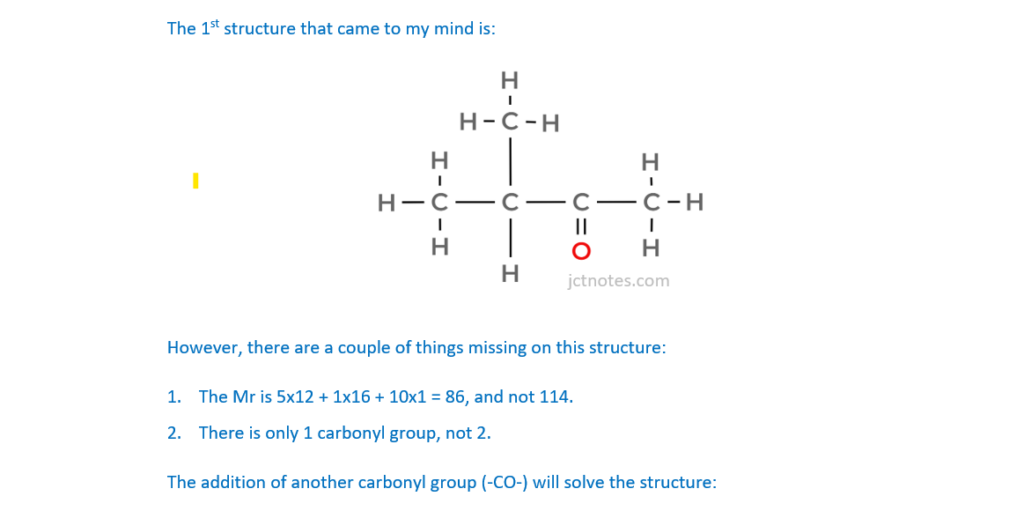
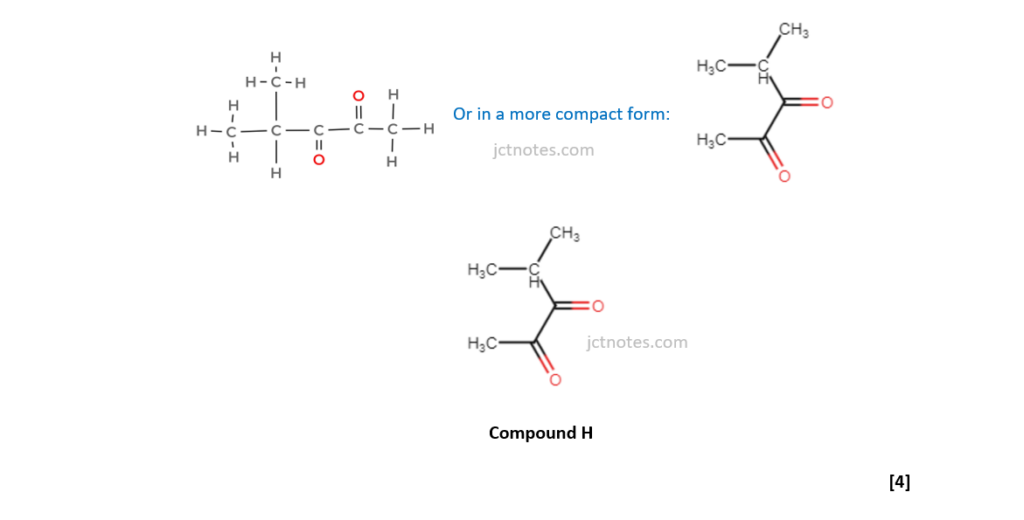
The organic compound H contains carbon, hydrogen and oxygen only and has an Mr of 114.0.
Compound H has two carbonyl groups and no other functional groups.
The 1H NMR spectrum of organic compound H is shown below.
The numbers by the peaks are the relative peak areas.

Analyse the spectrum to suggest a possible structure for compound H.
Show all your reasoning.



19
This question is about compounds that contain the carboxylic acid functional group.
(a)
Carboxylic acids react with alkalis, metals and carbonates to form salts.
Write full equations for the following three reactions.
Show structures for organic compounds.
• the reaction of propanoic acid with aqueous potassium hydroxide:
As acid + alkali → salt + water:
CH3-CH3-COOH + KOH → CH3-CH3-COOK + H2O
• the reaction of aqueous methanoic acid with magnesium:
As acid + metal → salt + hydrogen:
2HCOOH + Mg → (HCOO)2Mg + H2
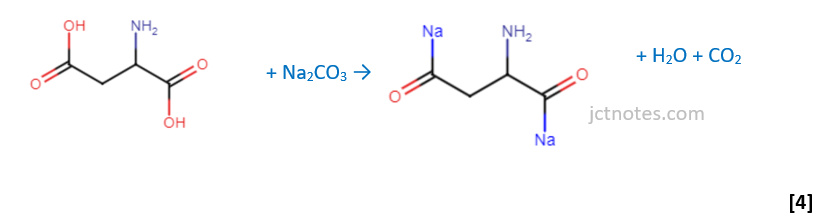
• the reaction of the α–amino acid, aspartic acid (R=CH2COOH), with an excess of aqueous sodium carbonate, Na2CO3:
As acid + carbonate → salt + CO2: I found the wording of the question is misleading, as it seems to ask the reaction of R=CH2COOH with Na2CO3, however, in the marking scheme they use the formula of the aspartic acid (HOOC-CH2-CH(NH2)-COOH) and the reaction is:
(b)
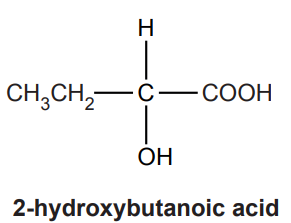
The structure of 2-hydroxybutanoic acid is shown below.
Fill in the flowchart for reactions involving 2-hydroxybutanoic acid.
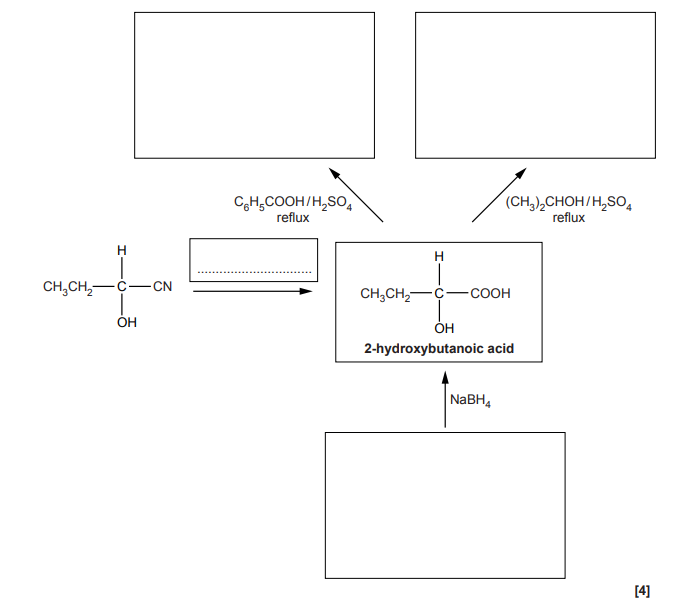
I have found the link OCR A Level Chemistry A – Topic Exploration Pack – Reaction pathways useful for this type of exercises.
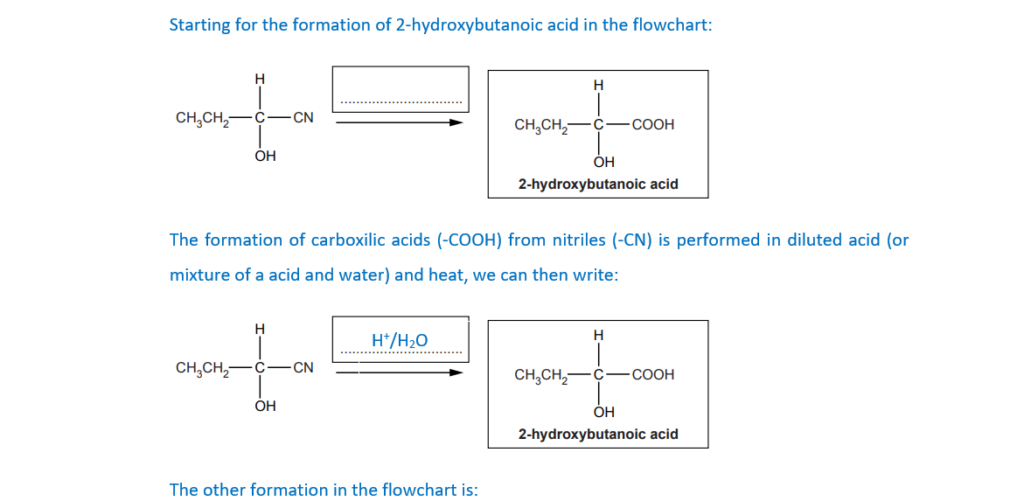
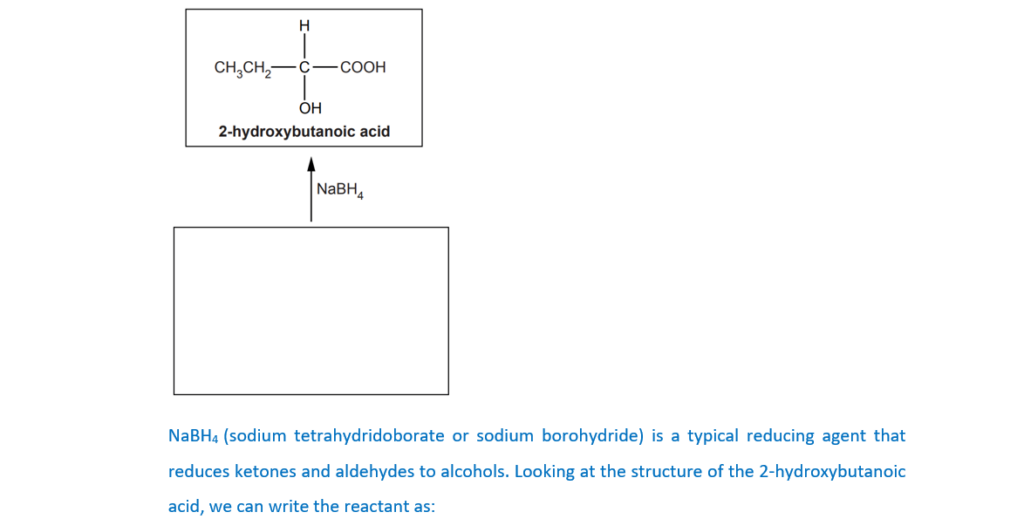
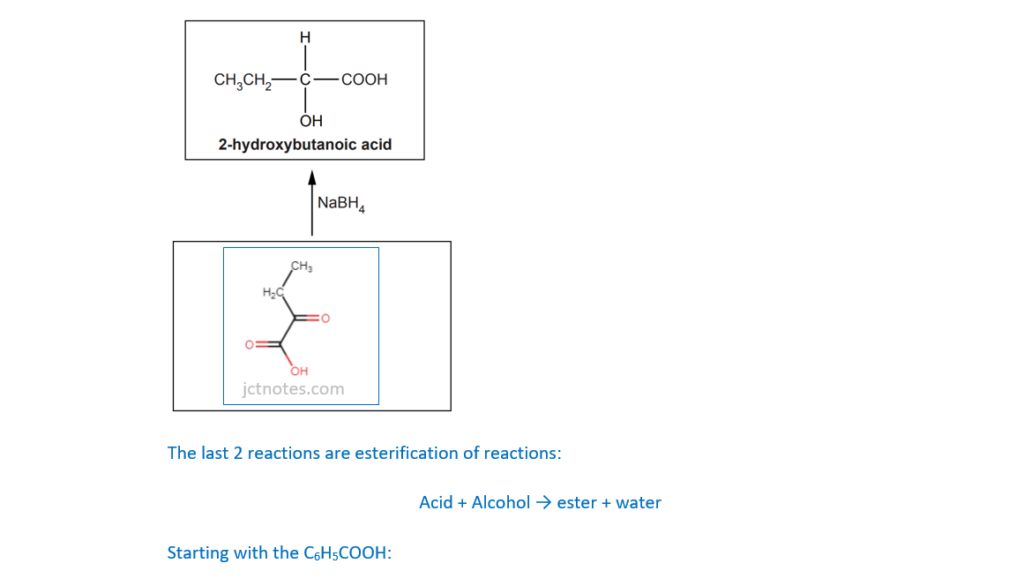
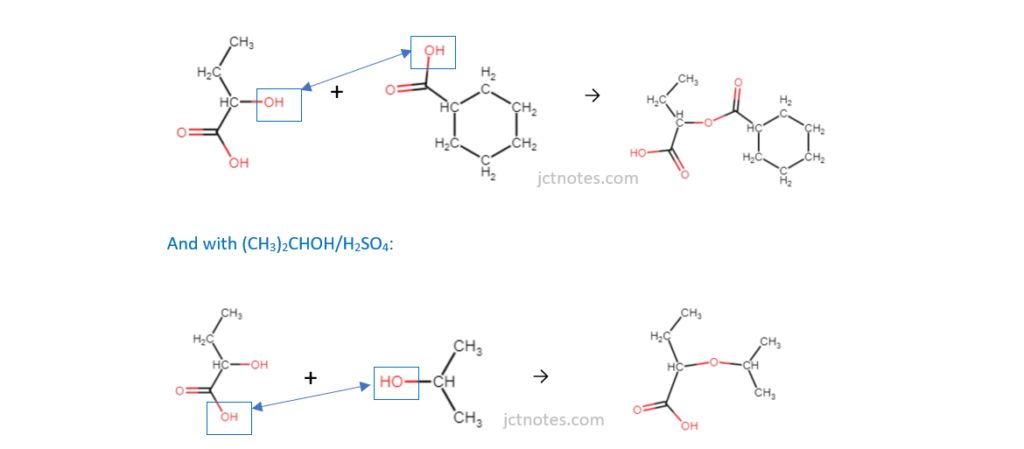
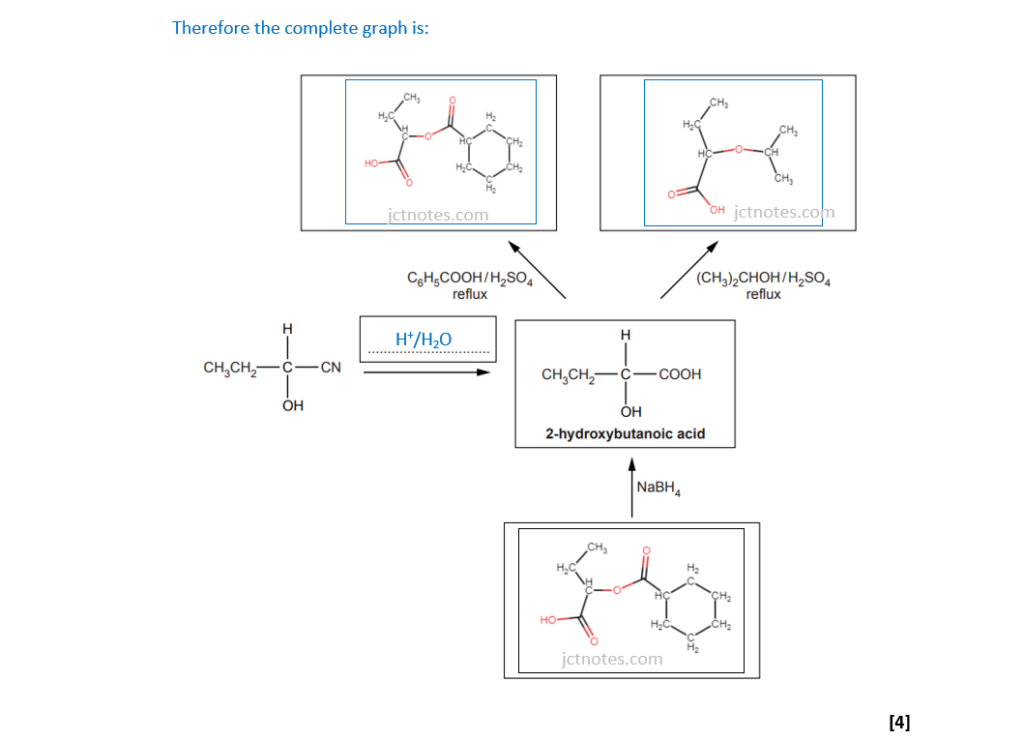
(c)
This part is about polymers derived from carboxylic acid monomers.
(i)
Poly(pent-3-enoic acid) is an addition polymer.
Draw the structure of pent-3-enoic acid and two repeat units of this polymer.
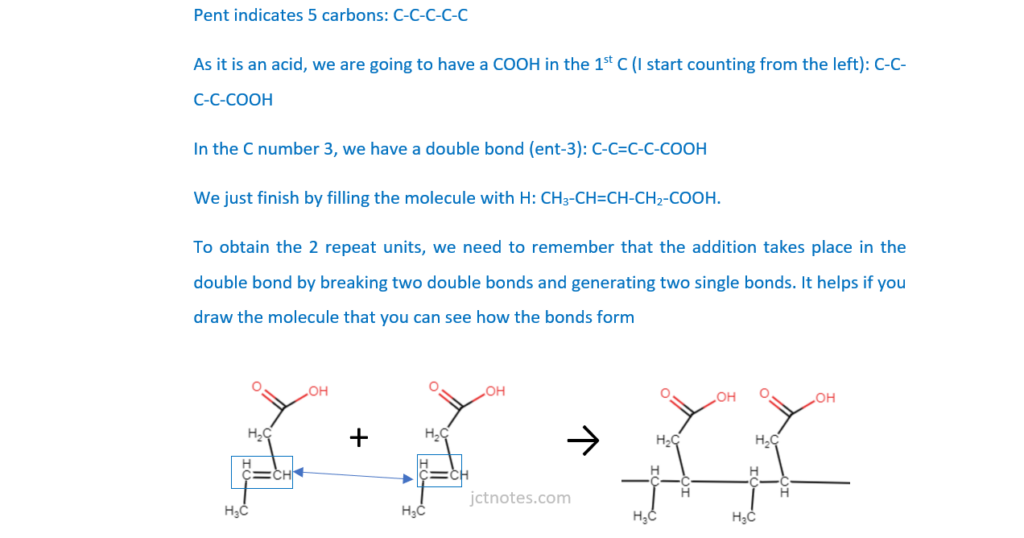
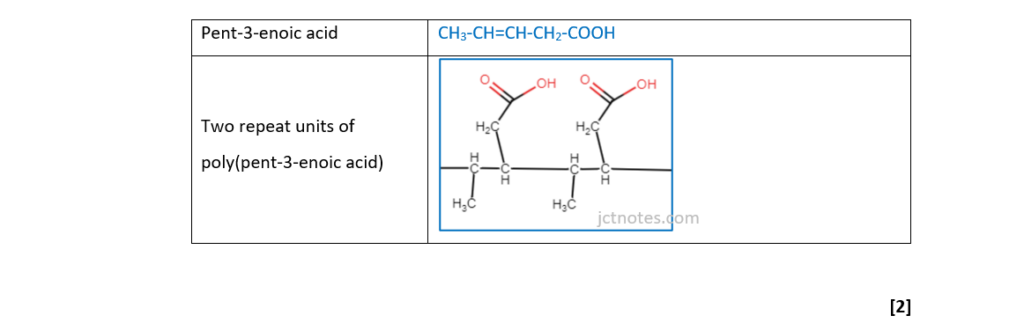
(ii)
Butanedicarboxylic acid and 1,4-dihydroxy-2-methylbenzene react to form a condensation polymer.
Draw one repeat unit of this condensation polymer.
[2]
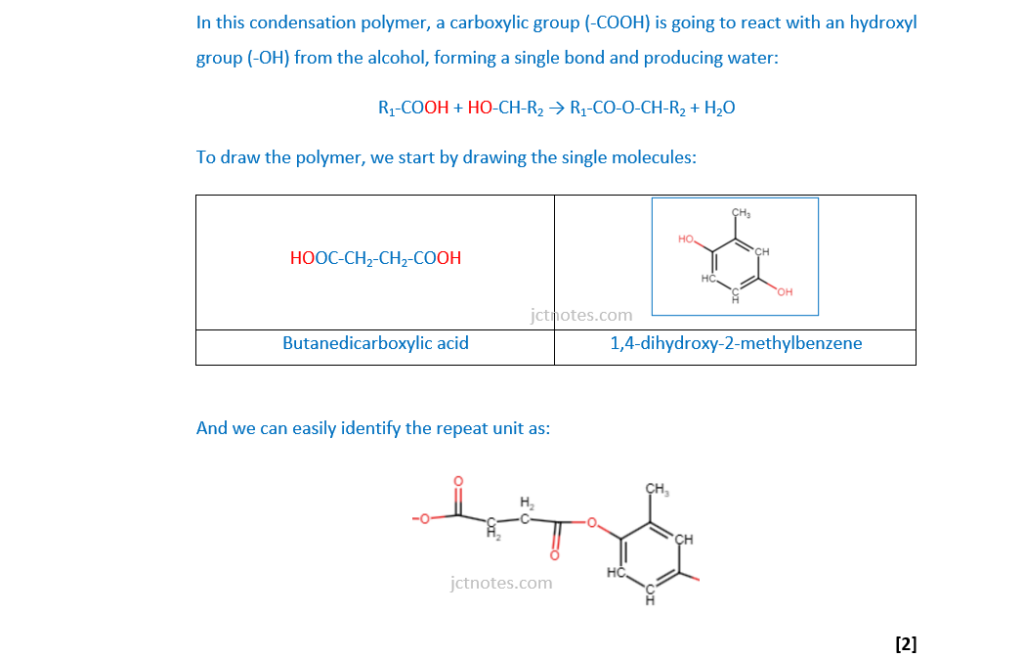
(iii)
Three repeat units of a condensation polymer are shown below.
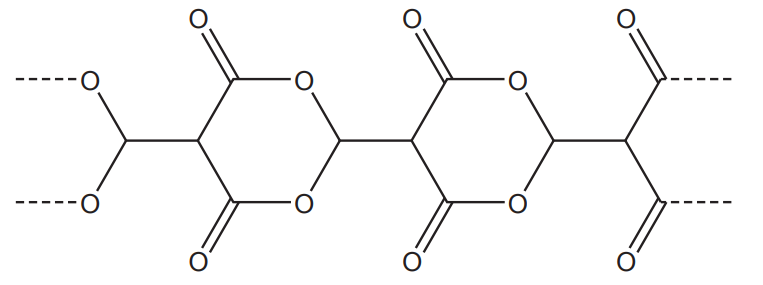
Draw the structure of the monomer required to form this polymer.
[1]
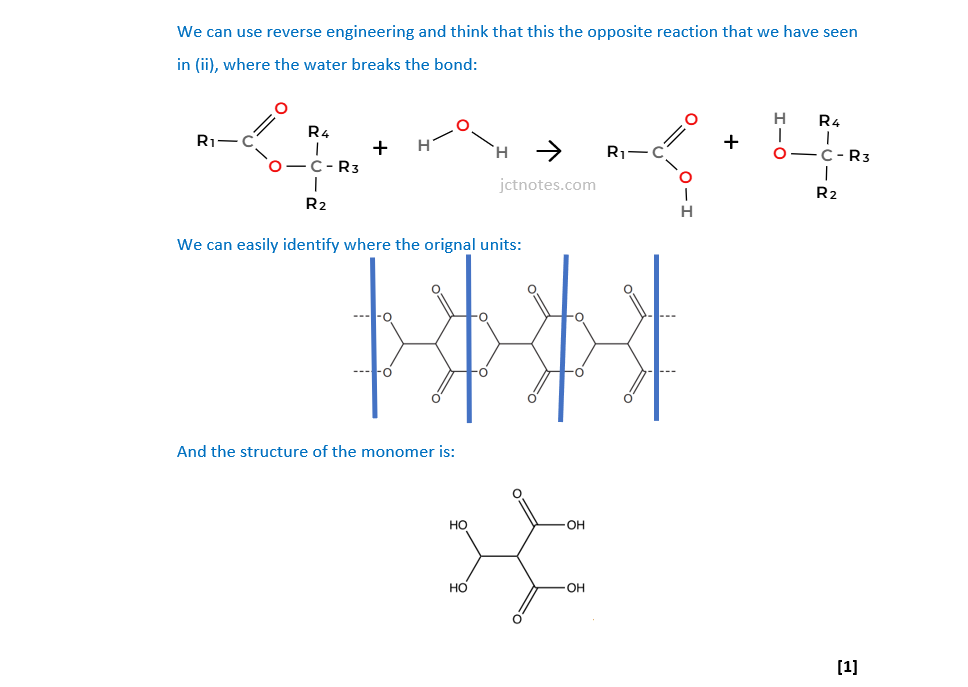
(d)
A polymer is formed from 400 molecules of 2-aminopropanoic acid.
(i)
Draw one repeat unit of this polymer.
[1]
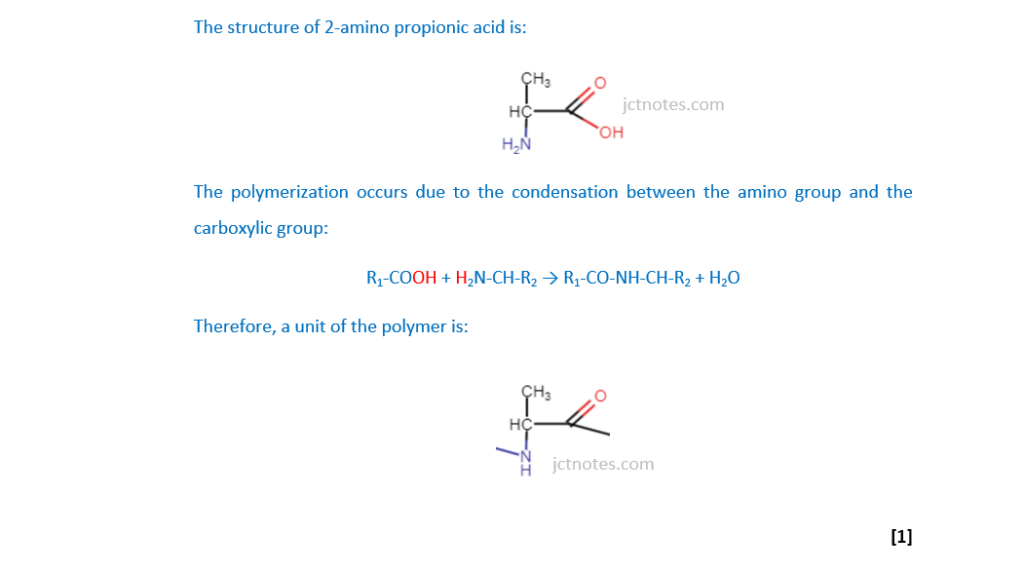
(ii)
What is the relative molecular mass, Mr, of the polymer?
The relative molecular mass of the 2-amino propionic acid (C3H7O2N) is 3×12 + 7×1 + 2×16 + 1×14 = 89.
The relative molecular mass of 400 molecules of 2-amino propionic acid is 89×400 = 35600. We have to account that in the condensation we are loosing 399 molecules of water, as each molecule of water has a relative molecular mass of 2×1 + 1×16 = 18, the lost of 399 molecules of water means the lost of 18×399 = 7182.
Therefore, the relative molecular mass of the polymer is 35600 – 7182 = 28418
Mr = ……………………28418……………………………[2]
(e)*
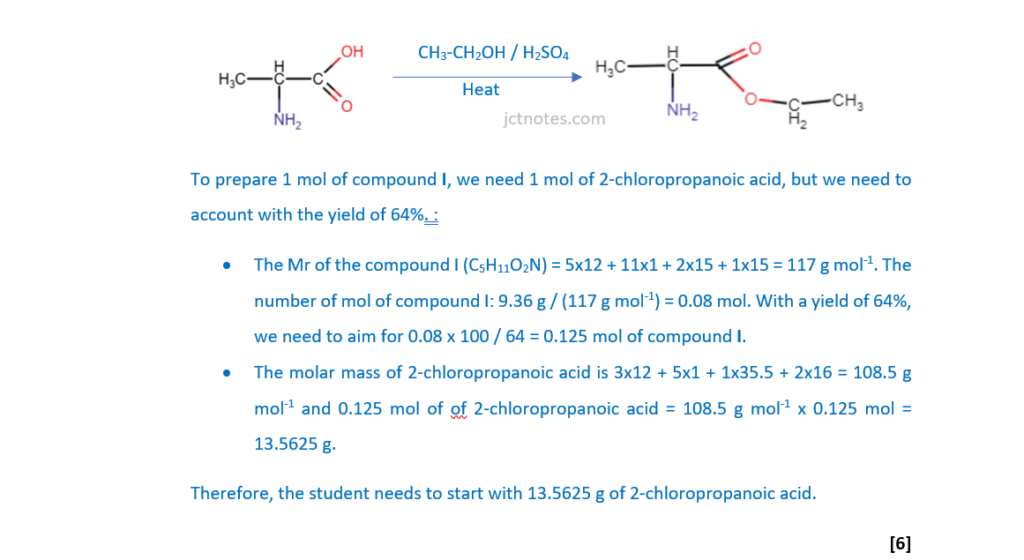
A student intends to synthesise compound I.
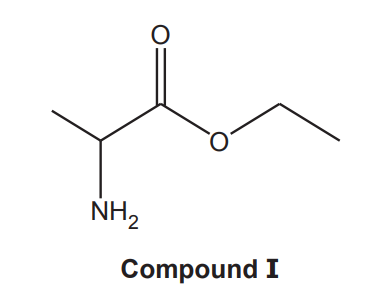
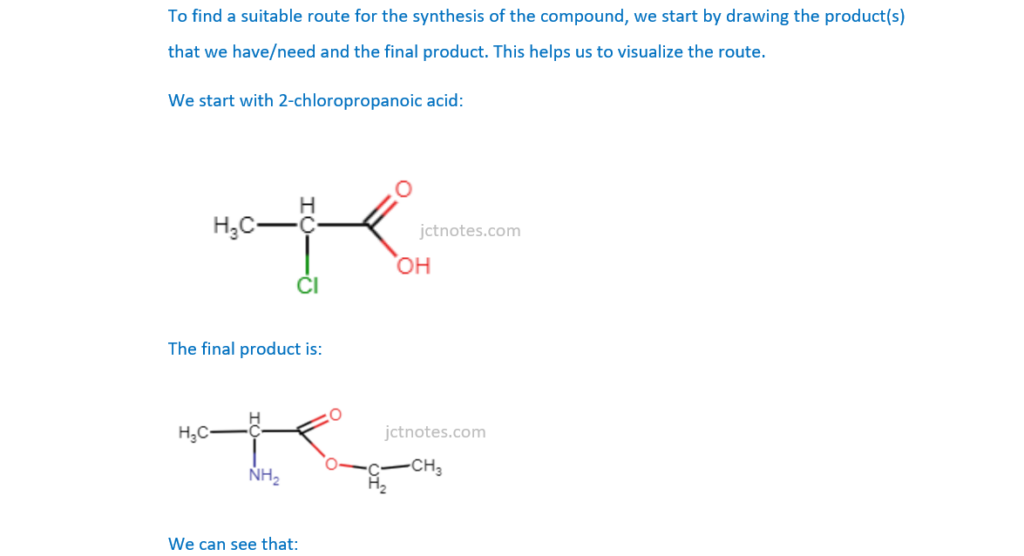
Plan a synthesis to prepare 9.36 g of compound I starting from 2-chloropropanoic acid, CH3CHClCOOH. The overall percentage yield of compound I from 2-chloropropanoic acid is 64%.
In your answer, include starting mass of 2-chloropropanoic acid, reagents, conditions and equations where appropriate.
[6]
Remember to have a look to OCR A Level Chemistry A – Topic Exploration Pack – Reaction pathways for these types of questions.
20
This question is about the chemistry of aromatic compounds.
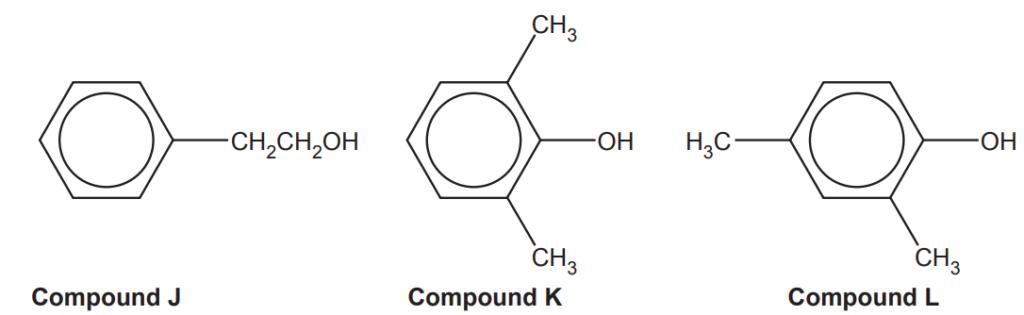
(i)
Compounds J, K and L, shown below, are structural isomers.
(ii)
What chemical test(s) could be used to confirm the presence of the phenol group in compounds K and L?
[1]

[1]
(ii)
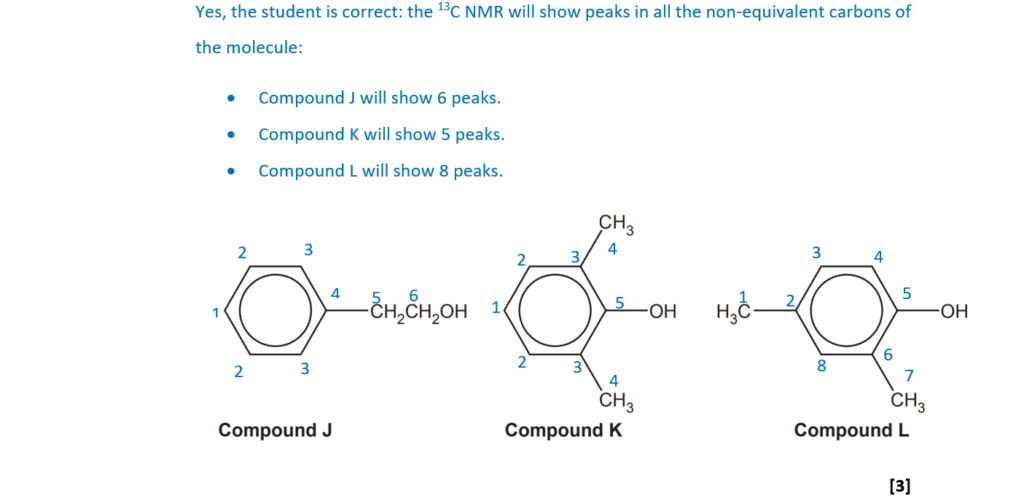
A student thought that 13C NMR spectroscopy could be used to distinguish between compounds J, K and L. Explain, with reasoning, whether the student is correct.
. [3]
(iii)
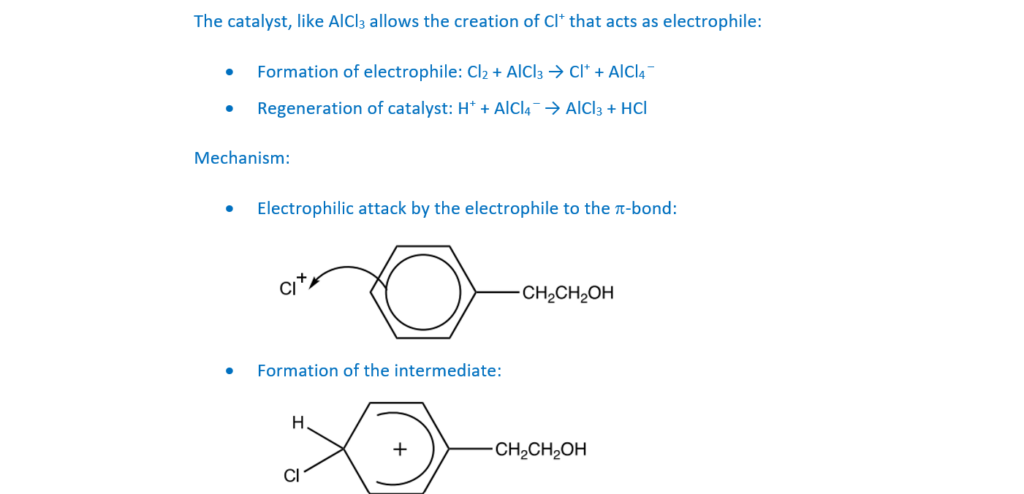
Compound J is substituted at the 2- and 4- positions by chlorine in the presence of a catalyst.
Outline the mechanism for the 4 substitution of compound J by chlorine in the presence of a catalyst.
Show the role of the catalyst.
[4]
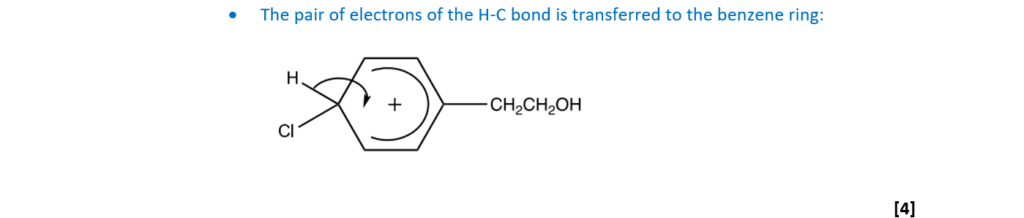
(b)
Compounds K and L react with chlorine much more readily than compound J.
Explain why.
[3]

(c)
Compound J, C6H5CH2CH2OH, is reacted with acidified potassium dichromate(VI) under reflux to form organic product M.
Write an equation for this reaction.
Use [O] to represent the oxidising agent and show the structure of M.
[2]
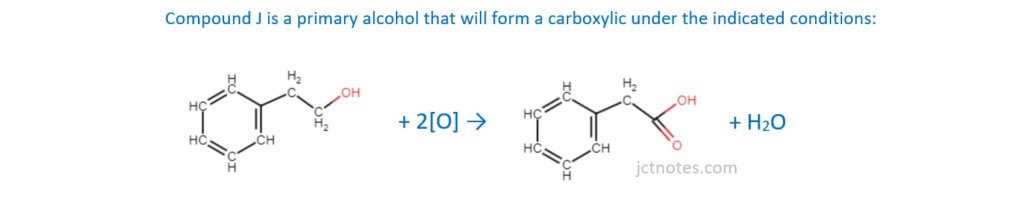
[2]
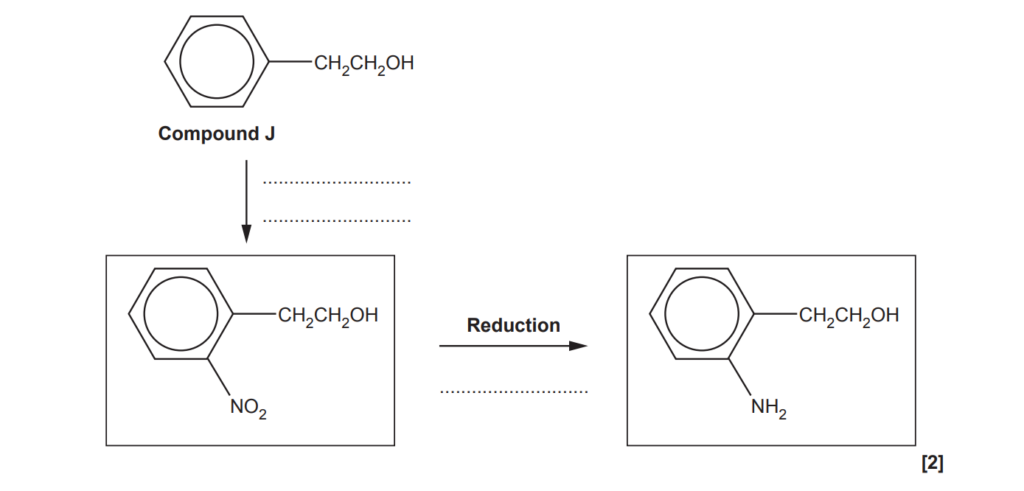
(d)
A two-stage synthesis of an amine from compound J is shown below.
(i)
Add the reagents for each stage of this synthesis.
The 1st step is the nitration of the benzene group performed in concentrated sulfuric acid and concentrated nitric acid under reflux. The alkyl group favours the nitration into the positions 2 and 4.
The 2nd step is the reduction of the nitro group to form an amino. This is the typical reaction to obtain phenylamine from nitrobenzene and it is performed by heating nitrobenzene with tin (Sn) and concentrated hydrochloric acid.
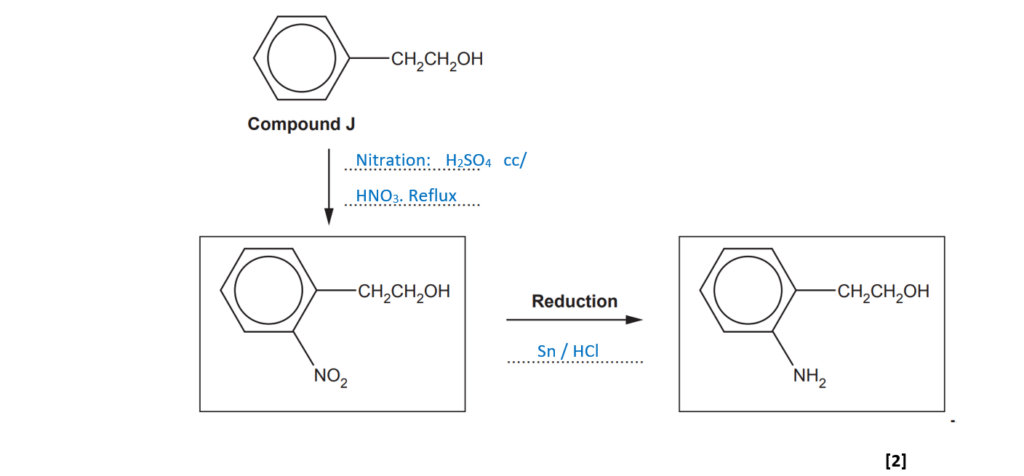
(ii)
Fill in the equation for the reduction stage of this synthesis.
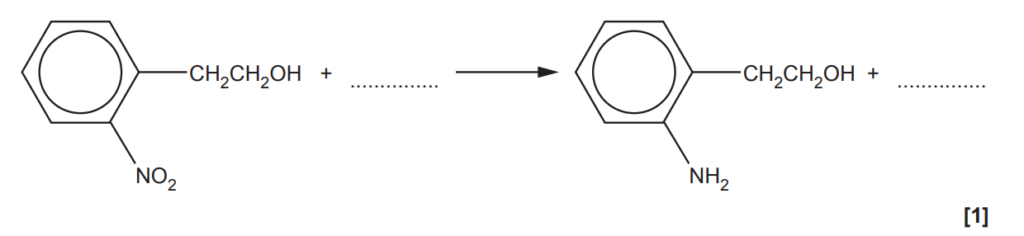
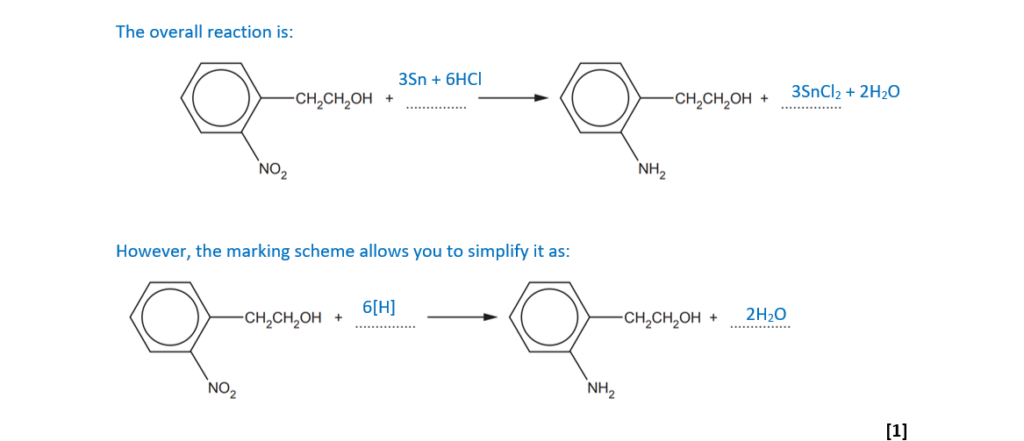
(e)
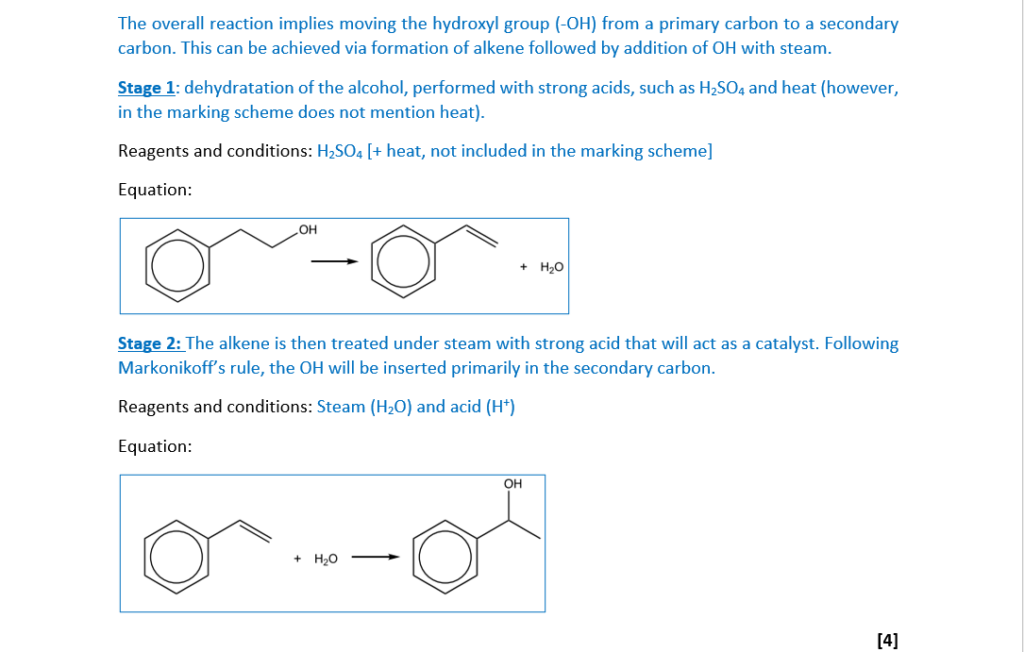
1-phenylethanol is a naturally occurring compound found in many vegetables and flowers.
1-phenylethanol can be synthesised from 2-phenylethanol in two stages.
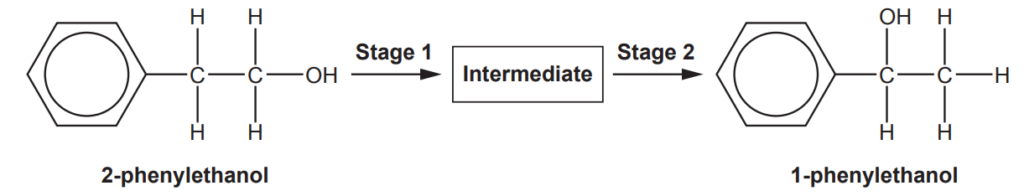
Suggest reagents, conditions and equations for each stage in the synthesis.
Show structures for organic compounds.
Stage 1
reagents and conditions ……………………………………………………………………………………………..
equation:
Stage 2
reagents and conditions ……………………………………………………………………………………………..
equation:
[4]
(f)
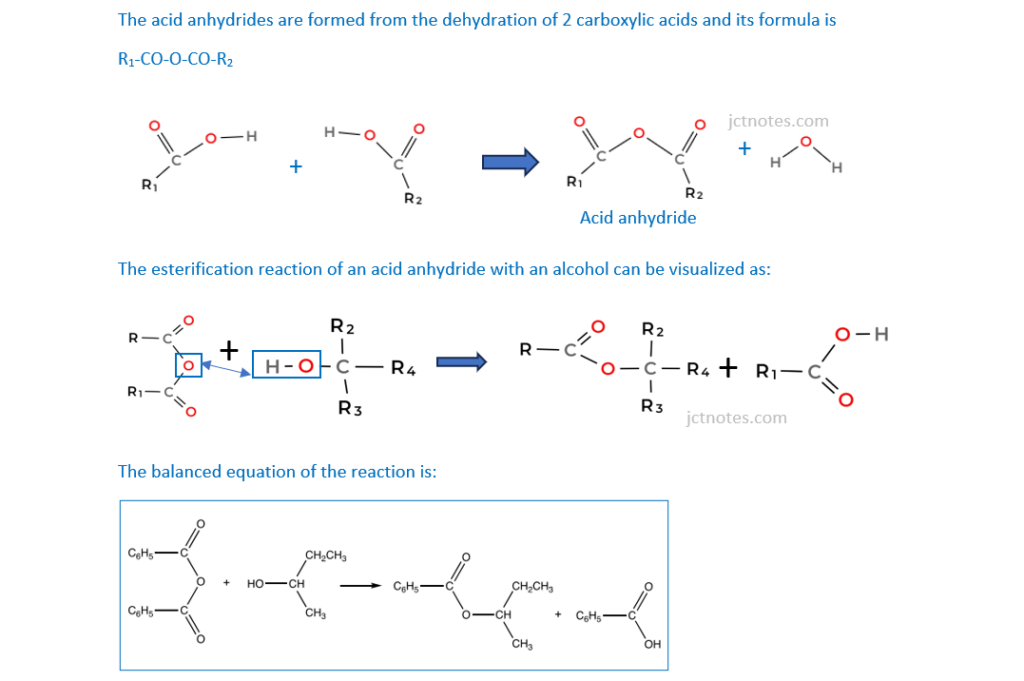
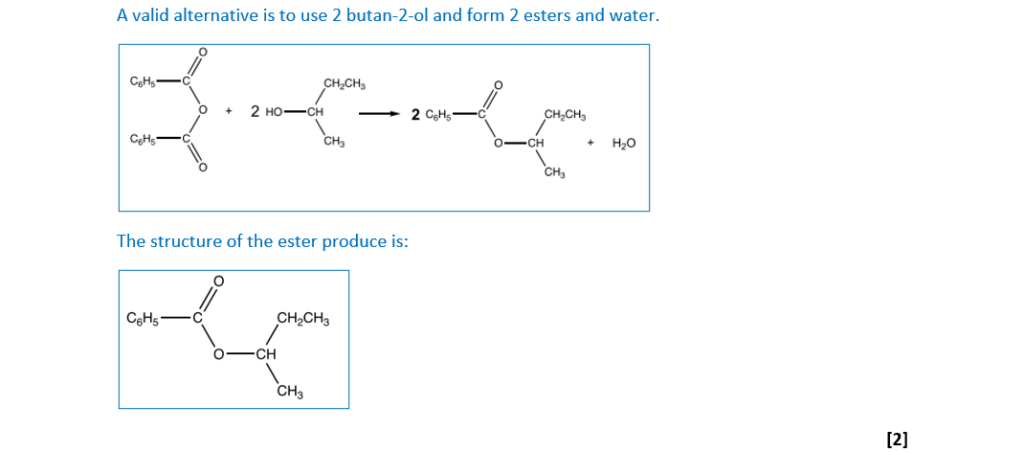
Acid anhydrides react in a similar way to acyl chlorides with phenols.
Benzoic anhydride is the acid anhydride of benzoic acid, C6H5COOH.
Benzoic anhydride reacts with butan-2-ol to form an ester.
Suggest an equation for this reaction. Show structures for organic compounds. Use C6H5 for any phenyl groups.
[2]
21*
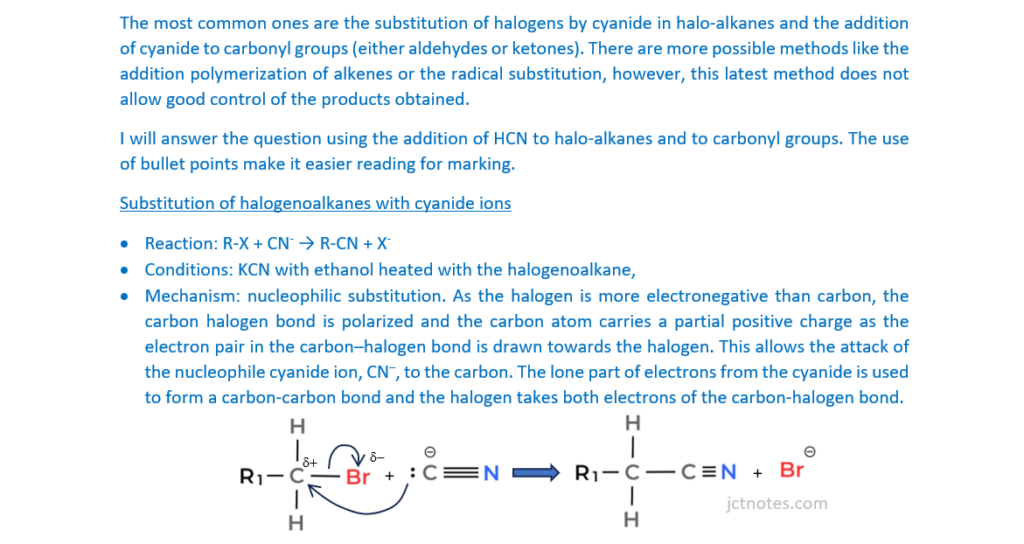
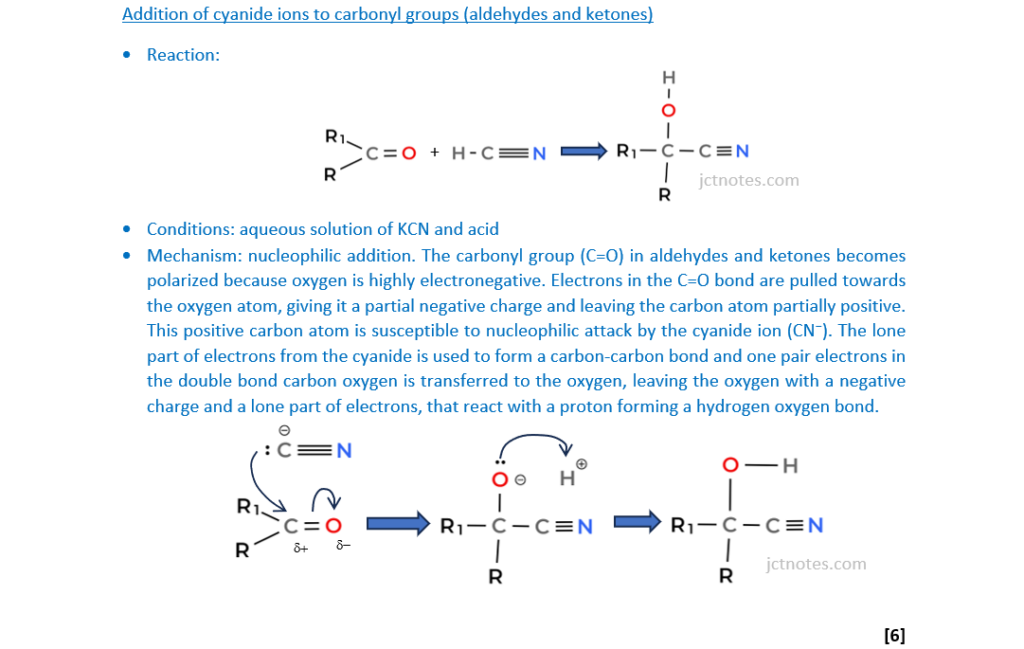
Carbon-carbon bond formation is used in synthesis to increase the length of a carbon chain.
Describe the formation of carbon–carbon bonds in aliphatic compounds by two different mechanisms.
Your answer should include mechanisms for each aliphatic compound.
[6]
END OF QUESTION PAPER
External links:
- Paper: H432-02_G_Jun22_301077.indd (ocr.org.uk)
- Mark scheme: Mark Scheme H432/02 Synthesis and analytical techniques June 2022 (ocr.org.uk)
- OCR data sheet for the exam: AS GCE (H032) A GCE (H432) Data Sheet for Chemistry A (ocr.org.uk)
- OCR reaction pathways OCR A Level Chemistry A – Topic Exploration Pack – Reaction pathways
