If you find this useful, please leave a comment at the end of the page.
A link to a video of this paper can be found in the link: Unlock Your AS Level Chemistry Potential: 2022 OCR Paper 1 H032/01 – Step-by-Step Solutions! (youtube.com)

SECTION A
You should spend a maximum of 25 minutes on this section.
Answer all the questions.
SECTION A
You should spend a maximum of 25 minutes on this section.
Answer all the questions.
Write your answer to each question in the box provided.
1. Which substance has a giant covalent lattice structure in its solid state?

2. What is the meaning of the term electronegativity?
A The ability of an atom to attract the electrons in a covalent bond.
B The ability of an atom to gain an electron.
C The electrostatic attraction between a negative ion and a positive ion.
D The size of the charge on a negative ion.
Your answer
[1]


4. What is the number of paired orbitals in a sulfur atom?

5, Which element has the lowest melting point?
A S
B P
C Cl
D Ar
Your answer
[1]

6. The first four ionisation energies of a Period 3 element X are shown in the table.

Element X is reacted with chlorine.
What is the formula of the chloride formed?
A XCl
B XCl2
C XCl3
D XCl4
Your answer
[1]

7,
A sample of lead(II) sulfate (M = 303.3g mol–1) is decomposed by heat, as shown in the equation
below.
2PbSO4(s) → 2PbSO3(s) + O2(g)
The reaction forms 2.40g of O2(g).
What is the mass of lead(II) sulfate that has been heated? Assume a 100% yield.
A 22.7g
B 30.3g
C 45.5g
D 60.7g
Your answer:
[1]
Using Factor label method:
2.40 g of O2 are:
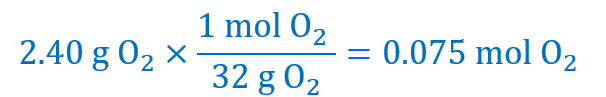
From the stoichiometry of the reaction, we can see that 0.075 mol of O2 are:

And 0.15 mol of PbSO4 are:

Your answer: C
8.
Which volume of 18.0 mol dm–3 hydrochloric acid should be diluted to 250.0 cm3 to prepare a
0.450 mol dm–3 solution of hydrochloric acid?
A 4.50 cm3
B 6.25 cm3
C 10.0 cm3
D 32.4cm3
Your answer
[1]
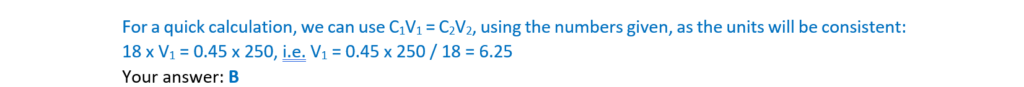
9.
What is the number of ions in 4.00mol of magnesium chloride, MgCl2?
A 1.81 × 1024
B 2.41 × 1024
C 4.82 × 1024
D 7.22 × 1024
Your answer
[1]
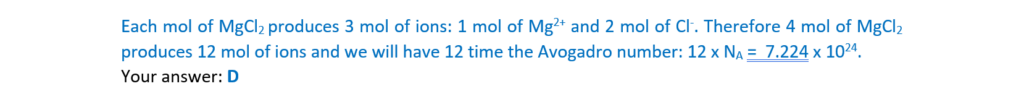
10.
What is the correct explanation for the trend in the boiling points of chlorine, bromine, and iodine
down the group?
A Bond enthalpy increases.
B Chemical reactivity decreases.
C Electronegativity decreases.
D London forces increase.
Your answer
[1]

11.
Combustion of hydrazine, N2H4, produces NO2 and H2O as in the equation below:
N2H4(l) + 3O2(g) → 2NO2(g) + 2H2O(l)
The table shows standard enthalpy changes of formation, ∆f Ho
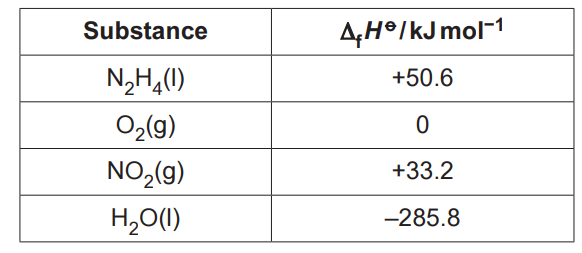
What is the enthalpy change of combustion, in kJmol–1, for hydrazine, N2H4(l)?
A –555.8
B –303.2
C +303.2
D +555.8
Your answer
[1]

12. Which prediction can be made using le Chatelier’s principle?
A The effect of a catalyst on the reaction rate.
B The effect of a catalyst on the equilibrium position.
C The effect of temperature on the reaction rate.
D The effect of concentration on the equilibrium position.
Your answer
[1]

13.
Which equilibrium has a numerical Kc value of 0.01?
Four equilibrium reactions are set up.
The concentration of each gas in the equilibrium mixtures is 0.1 mol dm–3.
A CH4(g) + 2H2O(g) ⇌ CO2(g) + 4H2(g)
B N2(g) + 3H2(g) ⇌ 2NH3(g)
C H2(g) + I2(g) ⇌ 2HI(g)
D 2NO2(g) ⇌ N2O4(g)
Your answer
[1]
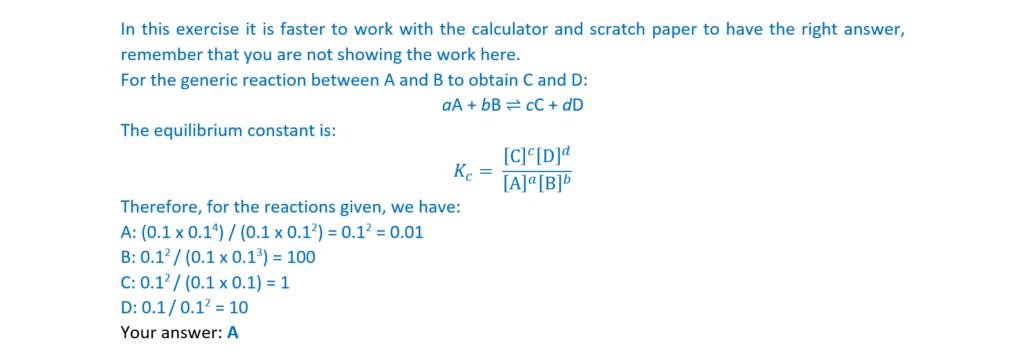
14.
What is the number of σ-bonds in the molecule below?
A 1
B 3
C 7
D 9
Your answer
[1]

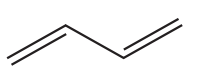
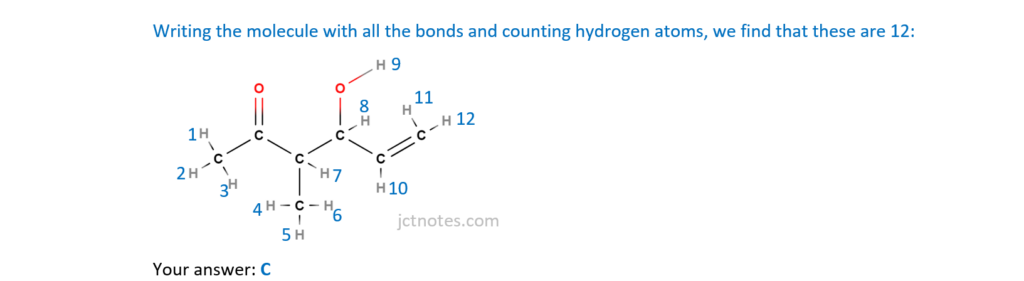
15.
What is the number of hydrogen atoms in one molecule of the compound below?
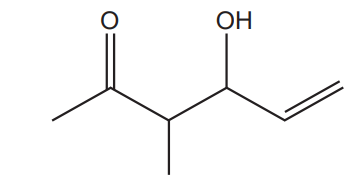
A 8
B 10
C 12
D 14
Your answer
[1]
16.
Complete combustion of an alkane forms 30 cm3 of carbon dioxide and 40 cm3 of water vapour, under the same conditions of temperature and pressure.
Which alkane has undergone complete combustion?
A butane
B ethane
C heptane
D propane
Your answer
[1]
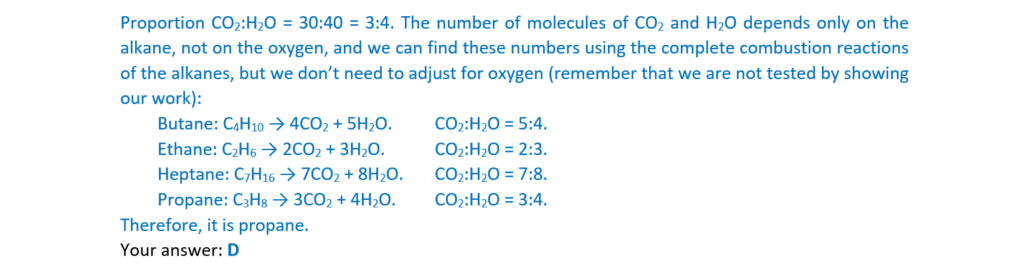
17.
Which alkene is an E stereoisomer?
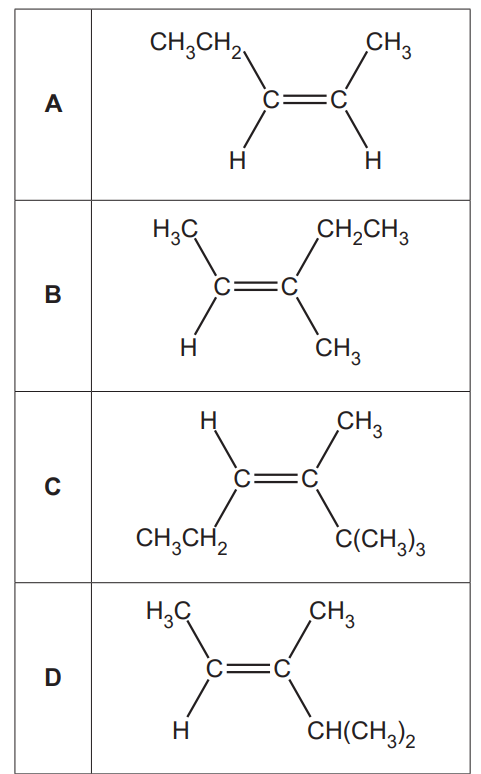
Your answer
[1]

18.
When heated with NaOH(aq), 1-chlorobutane is hydrolysed at a slower rate than 1-bromobutane.
Which statement explains the different rates?
A The C–Br bond enthalpy is greater than the C–Cl bond enthalpy.
B The C–Br bond enthalpy is less than the C–Cl bond enthalpy.
C The C–Br bond is less polar than the C–Cl bond.
D The C–Br bond is more polar than the C–Cl bond.
Your answer
[1]

19.
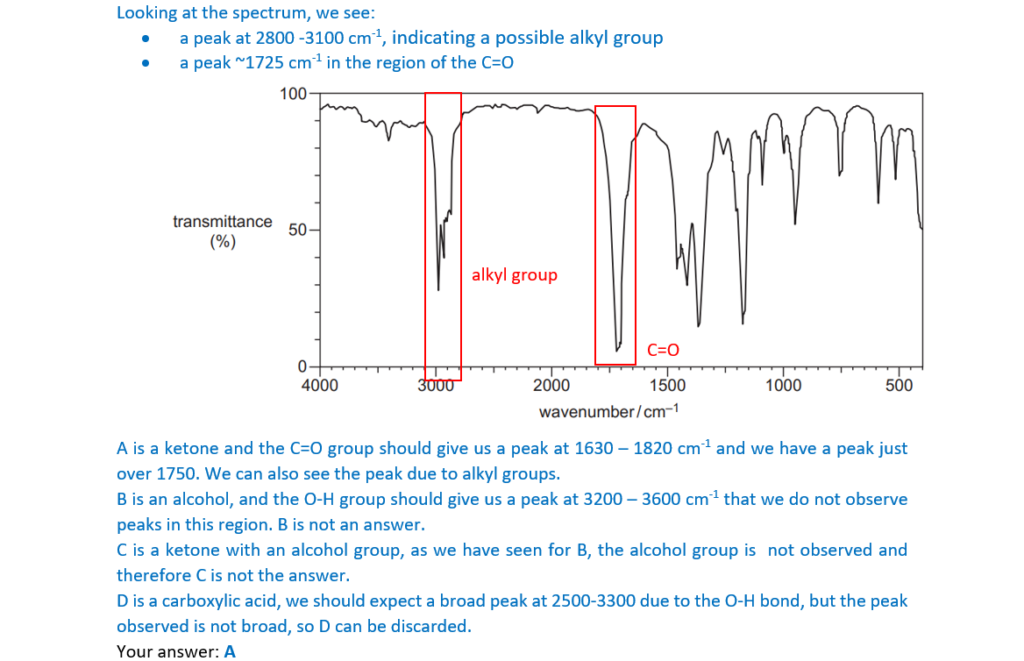
Which organic compound could have produced the infrared spectrum below?
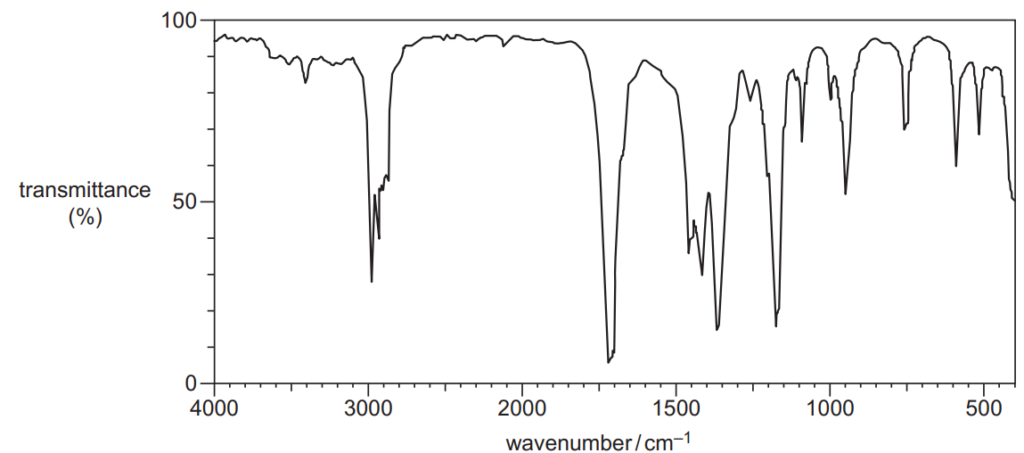
A CH3COCH2CH3
B CH3CH2CHOHCH3
C CH3COCH2CH2OH
D CH3CH2COOH
Your answer
[1]
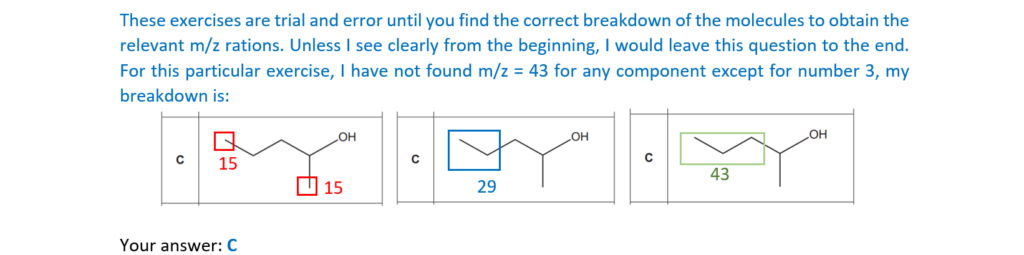
20.
Which alcohol is likely to have fragment ions at m/z = 15, 29 and 43 in its mass spectrum?
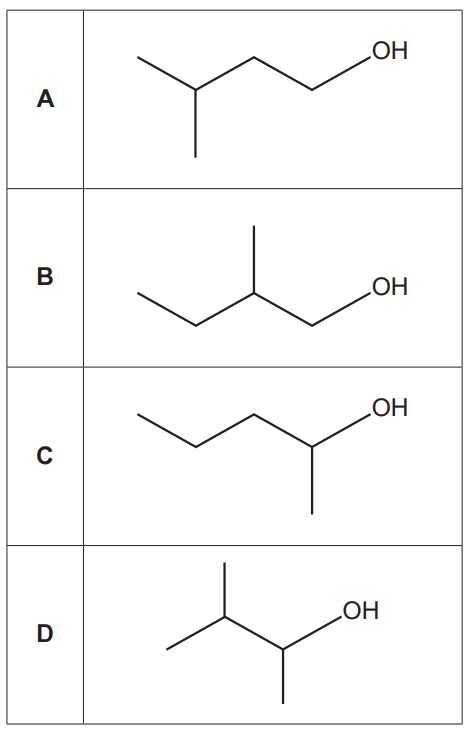
Your answer
[1]
SECTION B
Answer all the questions.
21.
The alkene, (CH3)3CCH=CH2, is used to make some perfumes.
(a)
(i)
What is the systematic name for (CH3)3CCH=CH2?
…………………………………………………………………………………………………………………….. [1]
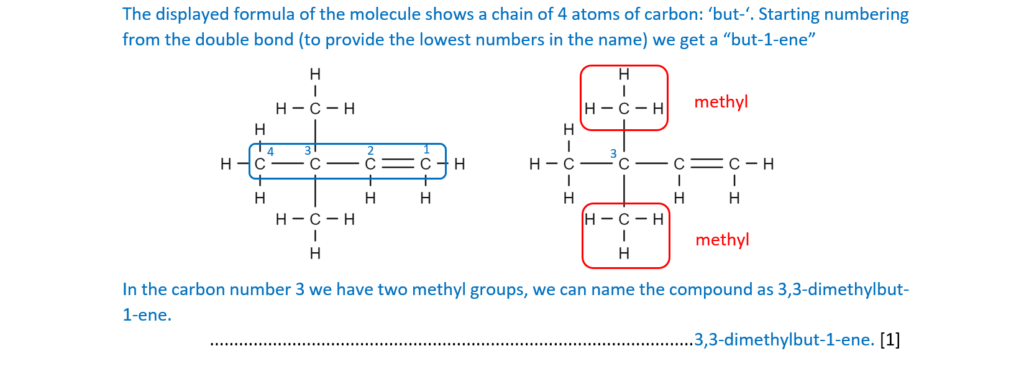
(ii)
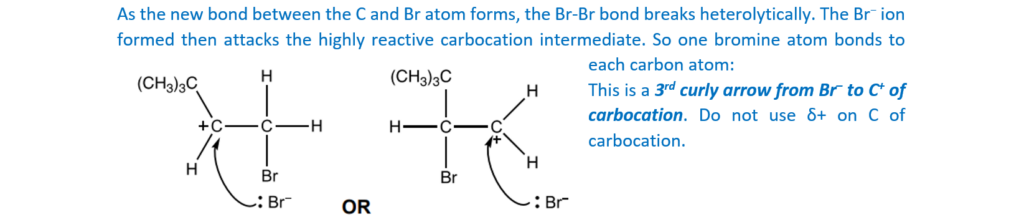
(CH3)3CCH=CH2 decolourises bromine.
Outline the reaction mechanism for the reaction of (CH3)3CCH=CH2 and bromine.
The structure of (CH3)3CCH=CH2 has been provided.
Include curly arrows and relevant dipoles, the structure of the product and the name of the mechanism.
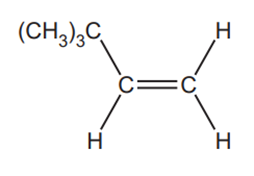
name of mechanism…………………………………………………………………………………………
[5]


(b)
The alkene (CH3)3CCH=CH2 can be polymerised to form a polymer.
(i)
Draw one repeat unit for this polymer.
[1]

(ii)
State one advantage and one disadvantage of using combustion as a method for the
disposal of a polymer after it has exceeded its useful life.
Advantage …Energy (or electricity) is produced ………………………………………………. ………………………………………………………………………………………………………………………….
Disadvantage …CO2 (or global warming gasses) are produced ……………….. ………………………………………………………………………………………………………………………[1]
22.
This question is about atomic structure and formulae.
(a)
The relative atomic mass of a sample of osmium can be determined from its mass spectrum,
shown below.
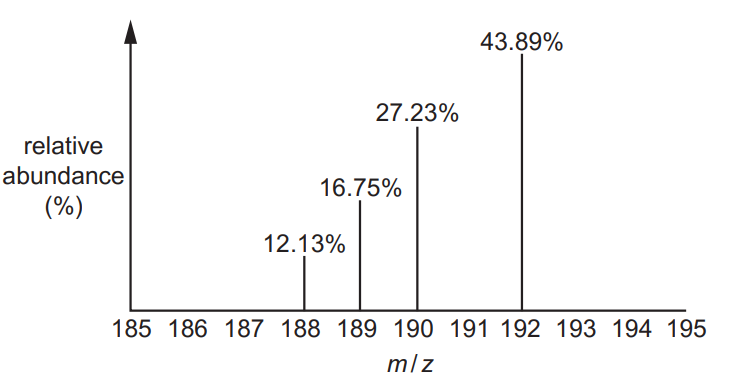
Calculate the relative atomic mass of osmium in the sample.
Give your answer to two decimal places.
relative atomic mass = ………………………………………………… [2]
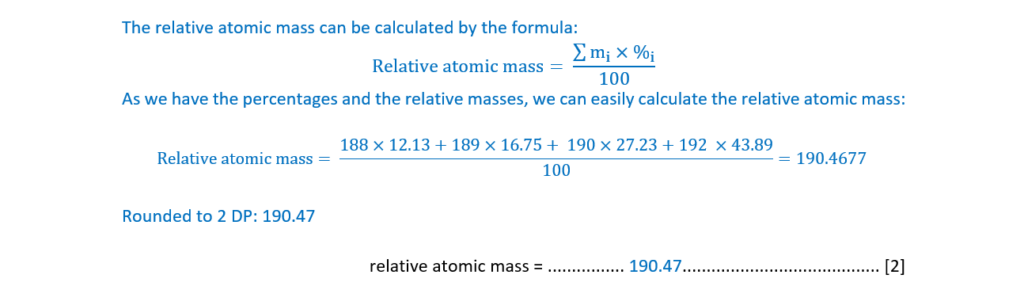
(b)
Complete the table for an atom and an ion of two different elements
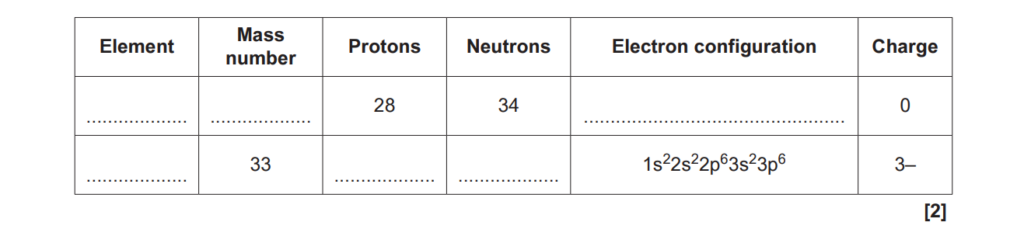
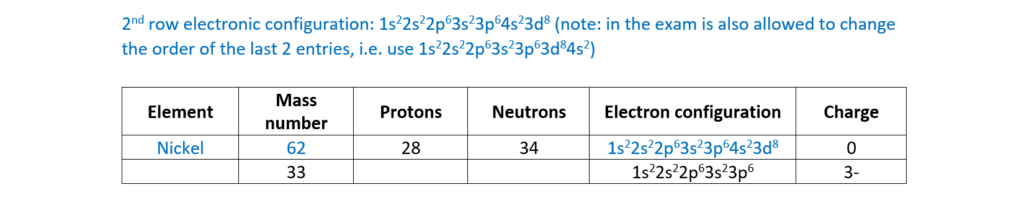
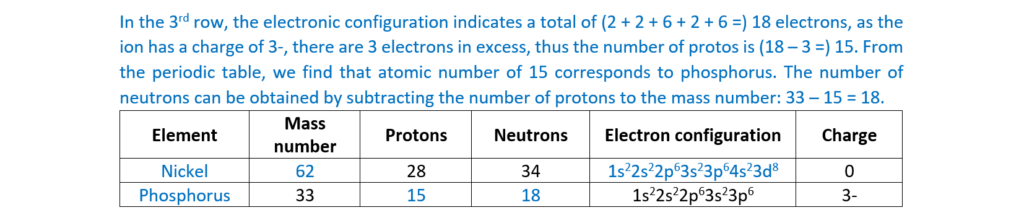
(c)
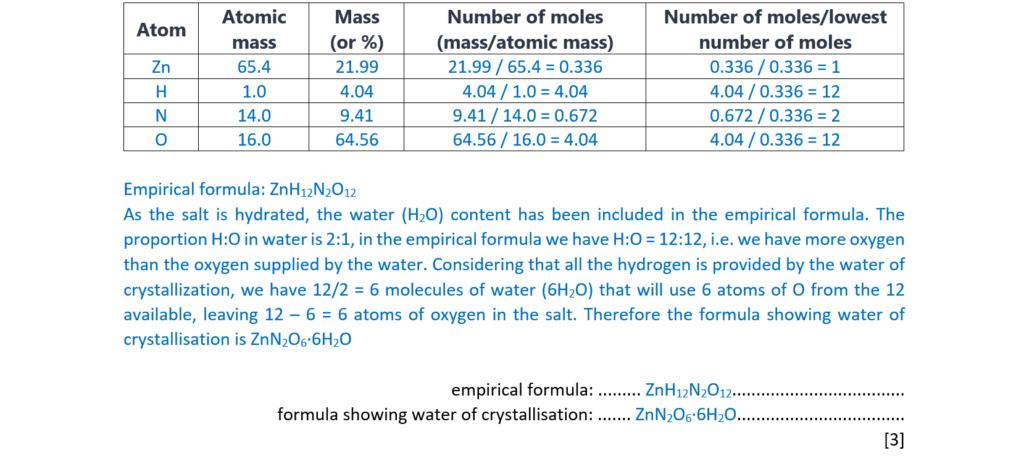
Substance A is a hydrated salt with the following percentage composition by mass:
Zn, 21.99%; H, 4.04%; N, 9.41%; O, 64.56%.
- Determine the empirical formula of A.
- Write the formula of A showing the water of crystallisation.
empirical formula: ………………………………………………………
formula showing water of crystallisation: ………………………………………………………
[3]
For an explanation about how to calculate empirical formulas and examples, check the following link: Formulas and formula masses – (jctnotes.com)
23.
This question is about different types of bonding
(a)
Ionic compounds have ionic bonding and exist in a giant ionic lattice structure
(i)
What is meant by ionic bonding?
……Ionic bonding: (Electrostatic) attraction between oppositely charged ions. …………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
……………………………………………………………………………………………………………………..
[1]
(ii)
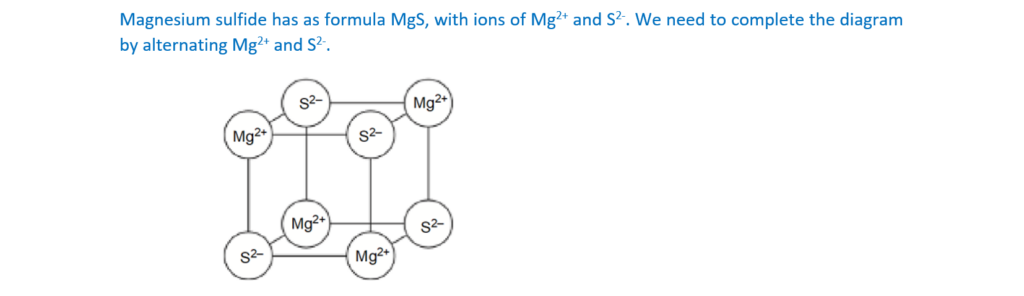
Magnesium reacts with sulfur to form a compound which has a giant ionic lattice
structure.
The diagram shows ions as circles in part of the lattice.
Complete the diagram by showing the symbols of the ions, including charges.
[2]
(b)
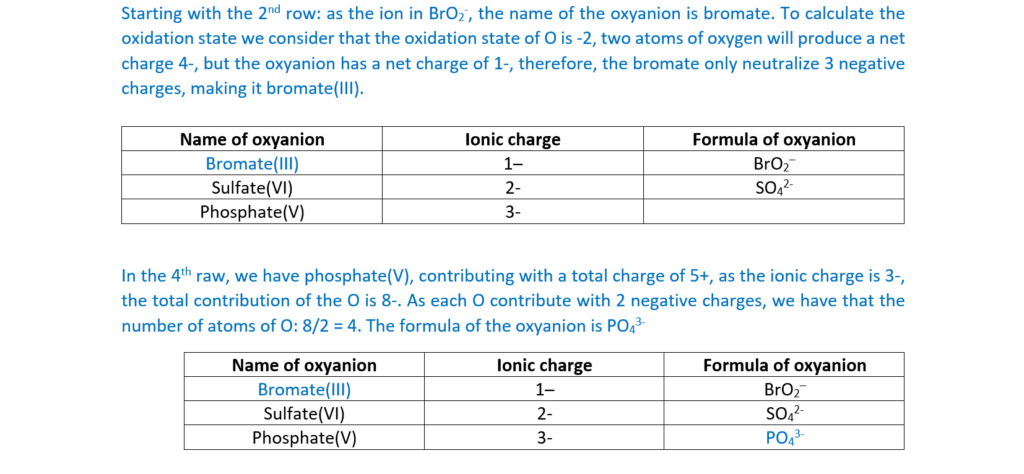
‘Oxyanions’ are ions containing oxygen combined with atoms of other elements.
Roman numerals are used to show the oxidation state of the element in the oxyanion.
Complete the table below for three oxyanions.
One row has been completed as an example.
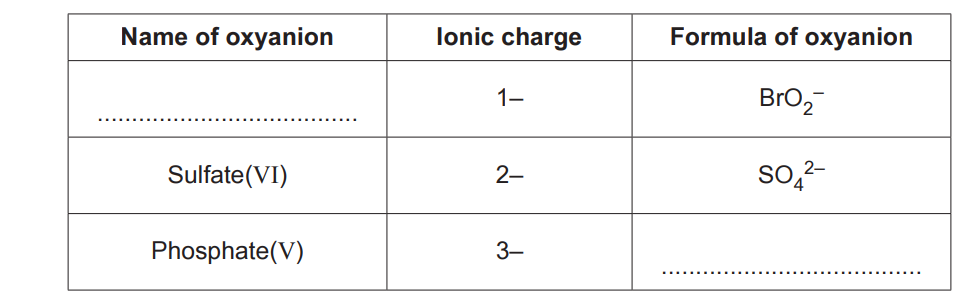
[2]
For explanation about how to calculate oxidation states, follow the link: How to balance redox equations – (jctnotes.com)
(c)
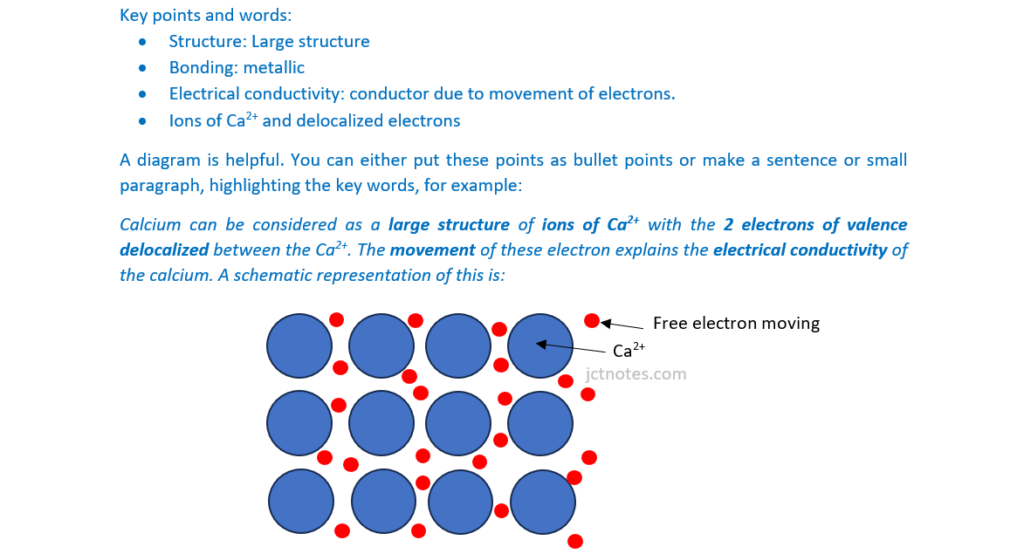
Describe the structure and bonding and electrical conductivity of calcium in the solid state.
You may wish to include a labelled diagram in your answer
[4]
24.
This question is about halogens and practical tests.
(a)
Chlorine gas reacts with dilute sodium hydroxide, NaOH(aq).
This is a disproportionation reaction. One of the products has the formula NaClO.
(i)
What is meant by the term disproportionation?
……………Oxidation and reduction of the same element………………………………
……………………………………………………………………………………………………………………..
[1]
(ii)
Construct the equation for the reaction of chlorine with dilute sodium hydroxide.
Use your equation to explain that disproportionation has taken place.
Equation …………………………………………………………………………………………………………….
Explanation ………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
…………………………………………………………………………………………………………………………
.
[3]

(b)
A student is supplied with aqueous solutions of ionic compounds B and C.
Compound B is a chloride, bromide or iodide of a Group 1 element.
Compound C is a chloride, bromide or iodide of a Group 2 element.
The molar masses of B and C are both in the range 100 – 115 g mol–1.
Use this information and test-tube tests to show how the student could identify the halide present in B and C and the formulae of B and C.
Explain your reasoning.
In your answer, include observations, colours and equations.
[5]

25.
This question is about enthalpy changes and reaction rates.
(a)
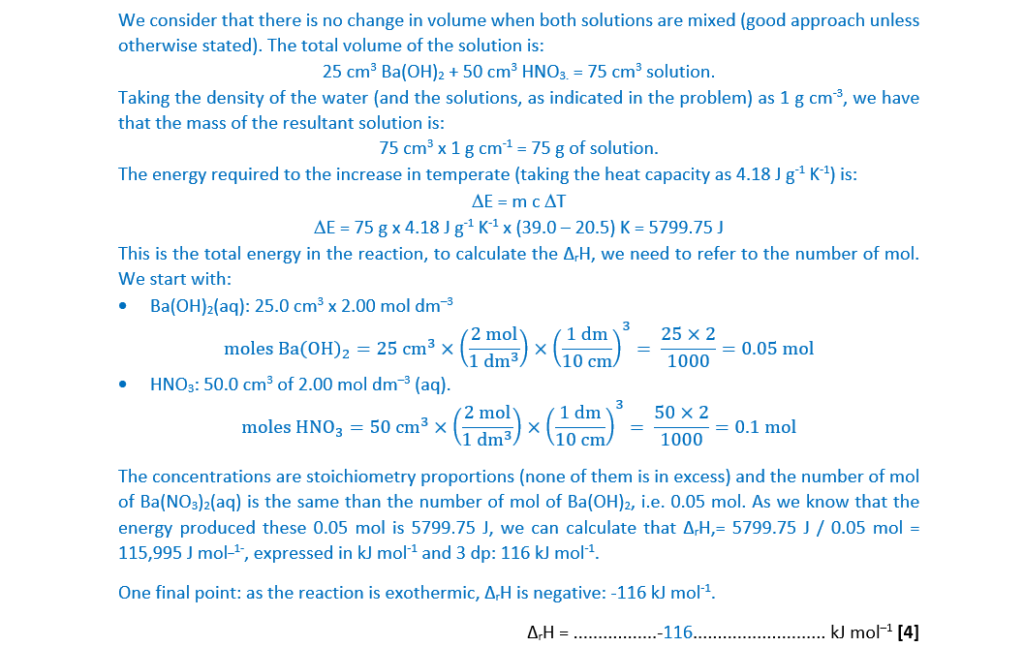
Aqueous barium hydroxide, Ba(OH)2(aq), reacts with dilute nitric acid, HNO3(aq), as in Equation 25.1.
Ba(OH)2(aq) + 2HNO3(aq) → Ba(NO3)2(aq) + 2H2O(l) ……………………… Equation 25.1
A student carries out an experiment to determine the enthalpy change of this reaction, ∆H.
The student measures out:
- 25.0 cm3 of 2.00 mol dm–3 Ba(OH)2(aq) and
- 50.0 cm3 of 2.00 mold m–3 HNO3(aq).
- The temperature of each solution is the same.
The student mixes both solutions in a polystyrene cup, stirs the mixture and records the maximum temperature.
Temperature readings:

(i)
Calculate ∆rH, in kJ mol–1, for the reaction shown in Equation 25.1.
Give your answer to 3 significant figures.
Assume that the density and specific heat capacity, c, of the solutions are the same as for water.
∆rH = …………………………………….. kJ mol–1
[4]
(ii)
The student looked back at Equation 25.1 and noticed that the reaction was a neutralisation.
The student concluded that ∆rH is the enthalpy change of neutralisation.
Explain why the student’s conclusion is incorrect and determine the correct value for the enthalpy change of neutralisation.
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
………………………………………………………………………………………………………………………….
enthalpy change of neutralisation = …………………………………….. kJ mol–1 [2]

(b)
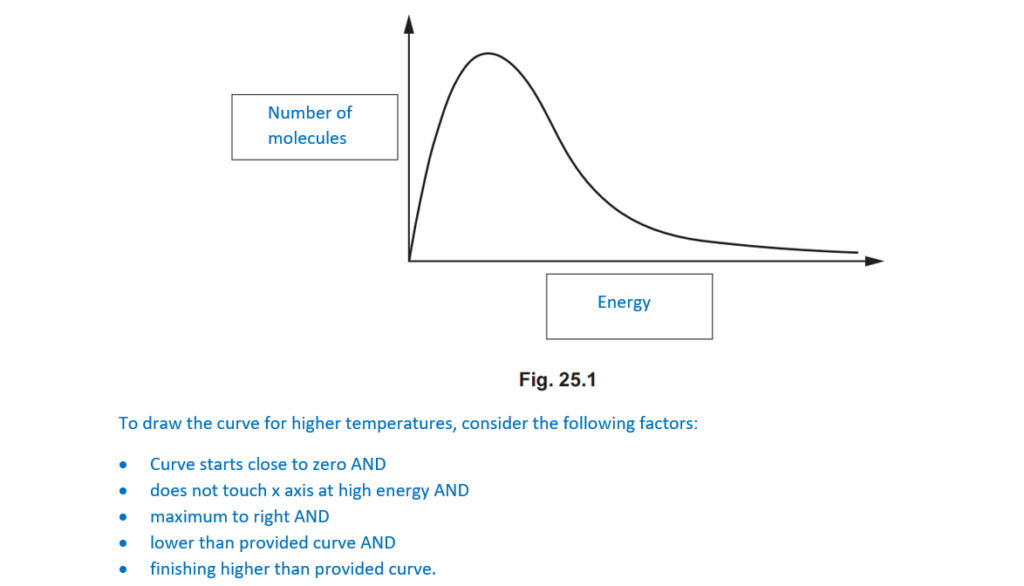
The Boltzmann distribution model can be used by chemists to explain how the rate of a reaction is affected by temperature.
Fig. 25.1 shows the Boltzmann distribution for a gas at room temperature.
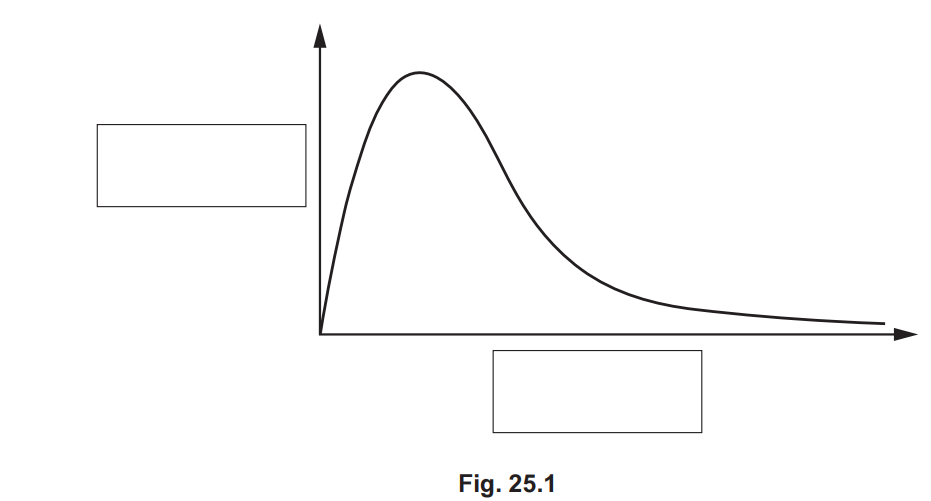
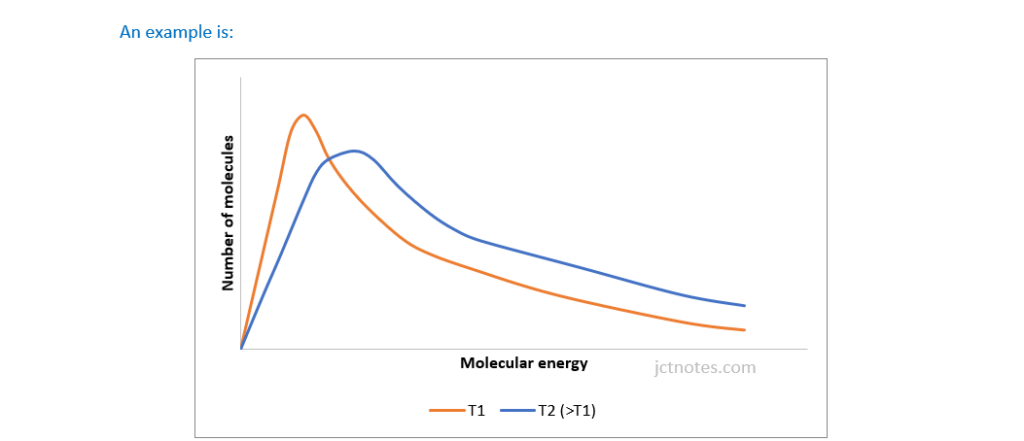
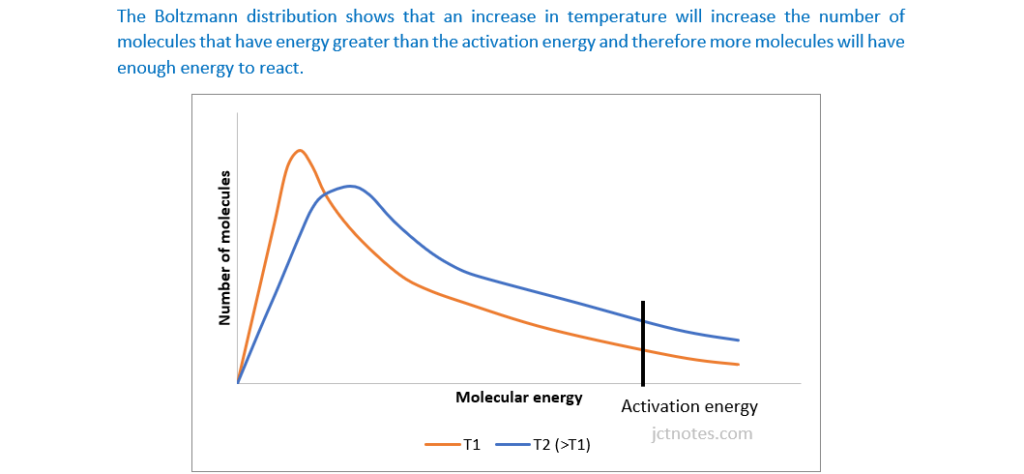
Label the axes on Fig. 25.1 and add a second curve to show the Boltzmann distribution of the gas at a higher temperature.
Explain why the Boltzmann distribution shows that the rate of a reaction is affected by temperature.
…………………………………………………………………………………………………………………………………
…………………………………………………………………………………………………………………………………
…………………………………………………………………………………………………………………………………
…………………………………………………………………………………………………………………………….
[3]
26.
This question is about haloalkanes.
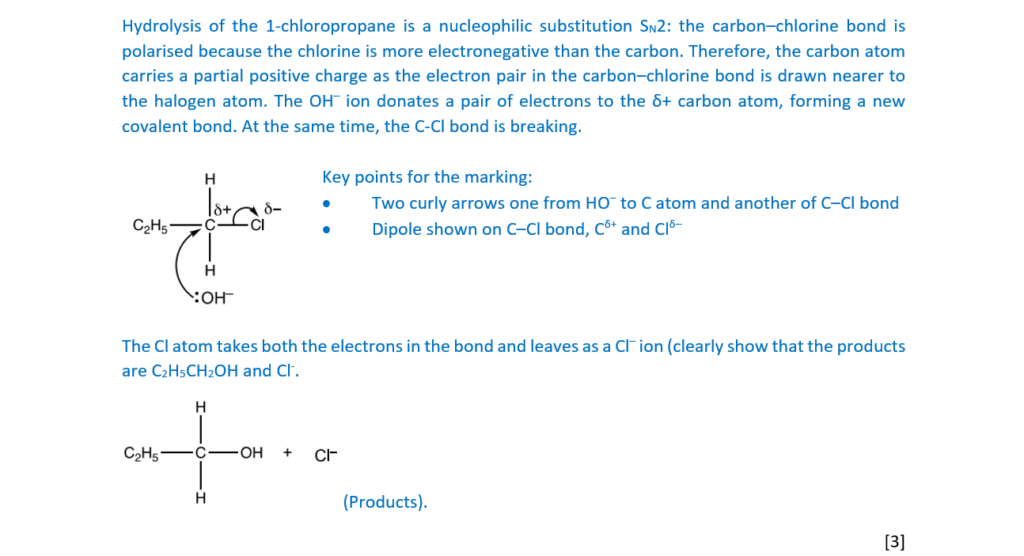
(a)
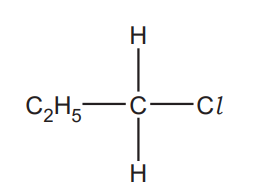
1-Chloropropane, C2H5CH2Cl, can be hydrolysed with aqueous sodium hydroxide, NaOH.
Outline the mechanism for this reaction.
The structure of 1-chloropropane has been provided.
Show curly arrows, relevant dipoles and product(s)
[3]
(b)
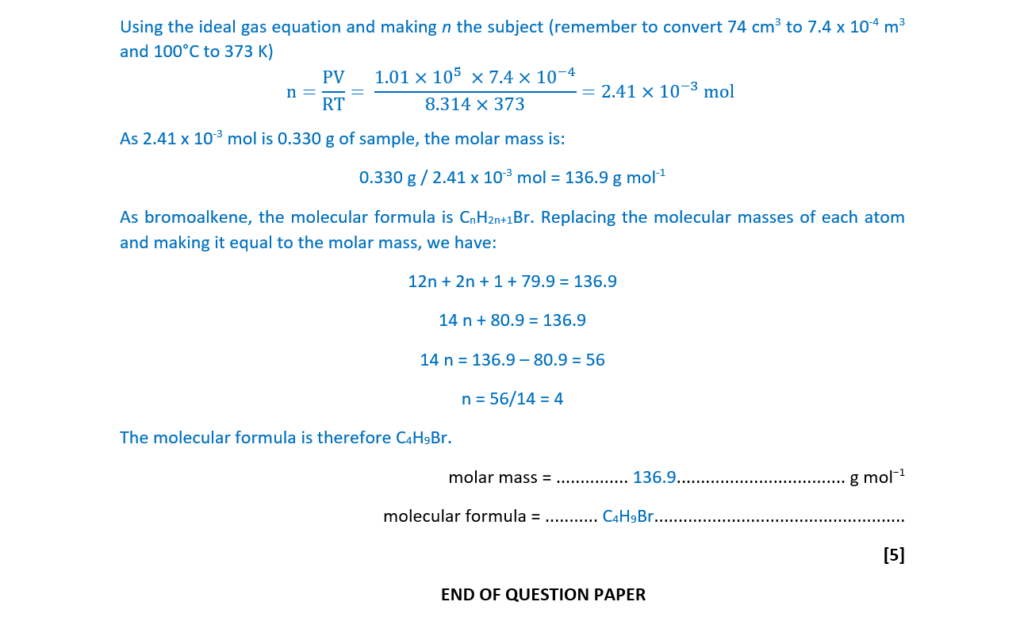
A bromoalkane D is a liquid at room temperature and pressure but can easily be vaporised.
When vaporised, 0.330 g of D produces 74.0 cm3 of gas at 1.01 × 105 Pa and 100°C.
Determine the molar mass and molecular formula of bromoalkane D.
molar mass = ………………………………………….. g mol−1
molecular formula = ………………………………………………………
[5]
External links:
- Paper: AS Level Chemistry A H032/01 June 2022 (ocr.org.uk)
- Mark scheme: Mark Scheme H032/01 Breadth in chemistry June 2022 (ocr.org.uk)
- OCR data sheet for the exam: AS GCE (H032) A GCE (H432) Data Sheet for Chemistry A (ocr.org.uk)
